« Prev Next »

Host Response to the Dengue Virus
Introduction
The Immune Response
Dengue Viral Infection
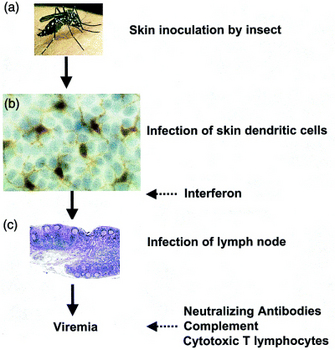
What happens when the mosquito Aedes aegypti infects someone with the dengue virus? When an infected mosquito feeds on a person, it injects the dengue virus into the bloodstream. The virus infects nearby skin cells called keratinocytes, the most common cell type in the skin. The dengue virus also infects and replicates inside a specialized immune cell located in the skin, a type of dendritic cell called a Langerhans cell.
What are Langerhans cells? Langerhans cells detect invading pathogens and display molecules from the pathogens, called antigens, on their surface. The Langerhans cells then travel to the lymph nodes and alert the immune system to trigger the immune response because a pathogen is in the body. Lymph nodes are small organs located throughout the body connected by vessels that form a network called the lymphatic system. The lymph nodes are stations in the body for immune cells that help fight against infections.
How the Dengue Virus Tricks the Immune System
How the Immune System Defeats the Dengue Virus
How can the body recover from a dengue infection? Although the dengue virus has tricked the immune system to infect cells and spread throughout the body, the immune system has additional defenses to fight the virus. The infected cells produce and release small proteins called interferons that are part of a large group of proteins called cytokines. Interferons have the ability to interfere with viral replication, and they activate both the innate and adaptive immune system defenses. They help the immune system recognize dengue-infected cells and help protect uninfected cells from infection. As the immune system fights the dengue infection, the person experiences a fever.
As the adaptive immune response starts fighting the dengue infection, B cells produce antibodies called IgM and IgG that are released in the blood and lymph fluid, where they specifically recognize and neutralize the dengue viral particles (Figure 2). In another adaptive immune response, cytotoxic T cells, or killer T cells, recognize and kill the cells that are infected with the dengue virus. The innate immune response activates the complement system, a response that helps the antibodies and white blood cells remove the virus. Together, the innate and adaptive immune responses neutralize the dengue infection, and the patient recovers from dengue fever.

Secondary Dengue Infections
After recovering from a first dengue infection, a person is protected from infection with the remaining three dengue serotypes for two to three months. Unfortunately, it is not long-term protection, and after that short period, a person can be infected with any of the remaining three dengue serotypes.
In the 1960s, Dr. Scott Halstead and his colleagues were studying the dengue virus in Thailand. They noticed that people who had been exposed to dengue a second time had an increased risk of severe dengue compared with those who had not been previously exposed. They wondered what makes a second dengue infection worse than the first.
Normally after an infection with a pathogen, the body "remembers" the infection for a long time because cells — called memory B cells and memory T cells — remain in the body. Because they remember the first infection, these memory cells can react rapidly to provide an adaptive response when an infection strikes a second time. Memory cells can remain in a person's body for many years, even an entire lifetime. Why, then, don't these memory cells help fight off a second dengue infection? Why is a second dengue infection often worse than the first infection?
Halstead proposed a phenomenon called "antibody-dependent enhancement of infection" to explain these observations. There are four different types of dengue viruses (serotypes), but the memory cells only provide immunity from reinfection with the dengue serotype that caused the first infection. When a person is infected with a second dengue serotype, Halstead proposed that antibodies from the first infection actually help spread the dengue viral infection and increase viremia, the amount of virus in the bloodstream. This phenomenon can also happen in children who received antibodies against dengue from their mothers while in the womb. Surprisingly, instead of destroying the virus, the existing antibodies and the antibodies newly produced by the memory B cells can actually help the virus infect host cells more efficiently (Figure 3). Ironically, the consequence of antibody-dependent enhancement is that the body's immune system response actually makes the clinical symptoms of dengue worse and raises the risk of severe dengue illnesses.

Researchers also observed that during a second infection with dengue, the cytotoxic T cells produced by the immune system provide only partial immunity against the new dengue serotype. The cytotoxic T cells do not effectively clear the virus from the body, and they release excess quantities of molecules called cytokines. In normal quantities, cytokines help the immune response; however, in high quantities, cytokines can produce serious inflammation and tissue damage such as leakage from the capillaries, possibly contributing to the development of severe dengue diseases.
Factors That Contribute to Severe Dengue Infections
Summary
References
Clarke, T. Dengue virus: Break-bone fever. Nature 416, 672–674 (2002). doi:10.1038/416672a
Diamond, M. S. Evasion of innate and adaptive immunity by flaviviruses. Immunology and Cell Biology 81, 196–206 (2003). doi:10.1046/j.1440-1711.2003.01157.x
Guzman, M. G. et al. Dengue: A continuing global threat. Nature Reviews Microbiology 8, S7–S16 (2010). doi:10.1038/nrmicro2460
Halstead, S. B. Dengue hemorrhagic fever: Two infections and antibody dependent enhancement, a brief history and personal memoir. Revista Cubana de Medicina Tropical 54, 171–179 (2002).
———. "Dengue: Overview and History." In Dengue: Tropical Medicine: Science and Practice, vol. 5, eds. G. Pasvol & S. L. Hoffman (London: Imperial College Press, 2008): 1–28.
Martina, B. E. E., Koraka, P., & Osterhaus, A. D. M. E. Dengue virus pathogenesis: An integrated view. Clinical Microbiology Reviews 22, 564–581 (2009). doi:10.1128/CMR.00035-09
Palucka, A. K. Dengue virus and dendritic cells. Nature Medicine 6, 748–749 (2000). doi:10.1038/77470.
Rothman, A. L. Dengue: Defining protective versus pathologic immunity. The Journal of Clinical Investigation 113, 946–951 (2004). doi:10.1172/JCI21512
Whitehead, S. S., et al. Prospects for a dengue virus vaccine. Nature Reviews Microbiology 5, 518–528 (2007). doi:10.1038/nrmicro1690
World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization and the Special Programme for Research and Training in Tropical Diseases, 2009.





















