« Prev Next »
The action of a single gene can have huge effects on how long a creature lives. This may seem hard to believe because so many things go into determining life span, including a host of lifestyle factors and a long list of diseases. Nonetheless, remarkable effects on life span are seen when particular genes are deleted from an animal's genetic sequence. Furthermore, research—particularly that involving microscopic roundworms—continues to provide scientists with tantalizing clues about the molecular pathways involved in aging.
Determination of the Aging Gene
When studying life span, scientists tend to work with organisms that do not live very long; that way, they can observe the entire course of an organism's existence and obtain relatively rapid experimental results. One organism that researchers frequently employ in their studies of life span is Caenorhabditis elegans, a microscopic roundworm that typically lives to a ripe old age of two to three weeks. Another advantage of using C. elegans is that these worms have a simple physiology and easily manipulated genes.
Over the last several decades, C. elegans has been the subject of many published studies, but perhaps the most famous of these appeared in 1993. In that paper, researcher Cynthia Kenyon and her associates showed that C. elegans with a specific single-gene mutation lived twice as long as members of the species that lacked this mutation (Kenyon et al., 1993). This finding was groundbreaking for a number of reasons. First, it challenged the prevailing concept that aging occurs as the body deteriorates over time. Second, it led to a shift in thinking, even among researchers who already believed that aging was subject to some sort of genetic control. Prior to this point, most such scientists figured that aging, age-related illnesses, and death were consequences of multiple cellular and physiological processes, and therefore under the regulation of a wide and diverse set of genes. Kenyon's paper, however, suggested that a single gene could dramatically regulate how long an organism lived, thus opening the door to new hypotheses about modifying life span through genetic manipulation.
The responsible gene is called daf-2, and, in 1997, a research group led by Gary Ruvkun finally solved its DNA sequence (Kimura et al., 1997). Scientists were surprised to find that the protein coded for by this gene (designated DAF-2) looked much like the receptor protein within humans that responds to the hormone insulin. In other words, the worm protein is simply a primitive form of our own insulin receptors.
Later research has revealed even more about daf-2. The gene is now known to regulate a number of factors in addition to aging, including stress resistance, metabolism, and development (Table 1) (Gami & Wolkow, 2006). Furthermore, scientists have determined that the gene and its hormone-signaling protein have been conserved evolutionarily, and they are found in many other animals, from fruit flies to mice. In worms and flies, the gene codes for a receptor protein that is activated by an "insulin-like" growth factor. This signaling pathway is analogous to the mammalian insulin pathway.
How the Aging Gene Works
So, how does a single gene cause such a dramatic effect? It turns out that daf-2 normally controls many other genes, which in turn regulate a variety of physiological processes at different stages in life. For example, in their studies of C. elegans, researchers have found a large set of genes that are either "turned on" or "turned off" in worms that carry two copies of the daf-2 mutation. The genes that show the most change fall into several different classes, some of which line up nicely with existing hypotheses about the mechanisms of aging in other organisms; this includes the belief that various genes encode for proteins that extend life by acting as antioxidants, regulating metabolism, and exerting an antibacterial effect.
| Turned On | Turned Off |
| Stress resistance Collagens Metabolism | Stress response Fertility Cell growth |
| Table 1. Functional classes of genes controlled by daf-2 Adapted from Gami & Wolkow 2006 | |
One particular gene affected by daf-2 is daf-16; this gene encodes a transcription factor, or a protein that determines when and where hundreds of other genes are turned on. Normally, the DAF-2 protein (which is an insulin receptor) exerts a dampening effect on the DAF-16 protein through phosphorylation, or the addition of a phosphate group. In the mutant worms, however, DAF-16 is not phosphorylated, and it is thus active and present in cell nuclei. Experiments have determined that this activation of DAF-16 (caused by the absence of a phosphate group) is a necessary step toward life span extension (Figure 1).
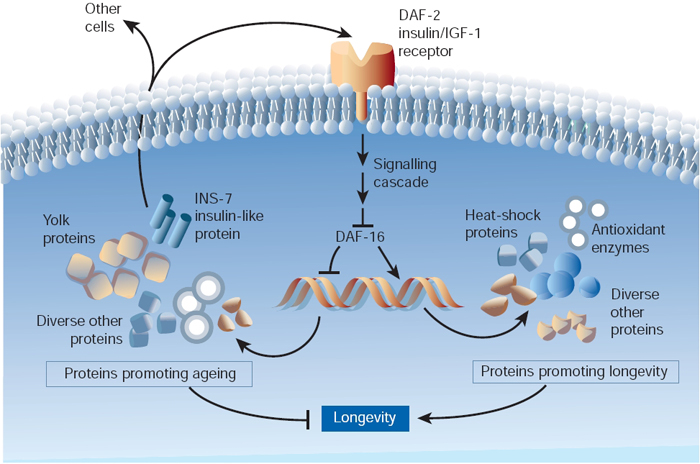
Why Limit Life Span?
Why, then, do animals have a gene such as daf-2, whose apparent purpose (when not mutated) is to limit life span? Try considering that question from a different point of view. The daf-2 gene seems to be just one cog in an extended genetic system that allows worms to regulate their development according to their circumstance. Thanks to this system, those aspects of growth and development that are necessary for reproduction can be put on hold if environmental conditions are poor. Indeed, growing worms are actually able to suspend their own development in a phase known as dauer. This allows them to wait—for months, if need be—for better times. Long before Kenyon's work, other researchers linked daf-2 and daf-16 to this arrested form of development. In fact, the genes' names derive from the phrase "dauer formation." Moreover, experimentally "knocking out" the activity of daf-2 sends a developing worm right into the dauer state, whether or not nutrients are scarce.
But is this sort of insulin signaling critically involved in longevity in humans as well? Scientists take that question seriously for a number of reasons. One clue to the hormone pathway's importance is that it has been conserved through evolution, a fact that always interests scientists because it is akin to a vote of confidence from Mother Nature. Another clue is the role that the insulin pathway plays in diseases like diabetes and cancer. For example, insulin resistance at the cellular level is a key feature of type II diabetes. Similarly, mutations along the pathway that insulin/IGF-1 receptors put into motion have been associated with the dysregulation of growth that characterizes cancer. The disease idea is especially tantalizing, as the risk of both diabetes and cancer increases with age.
So, how does one gene control life span? Quite simply, it acts by controlling a lot of other genes that just happen to coordinate the survival system within worms.
References and Recommended Reading
Gami, M. S., & Wolkow, C. A. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of life span. Aging Cell 5, 31–37 (2006) doi:10.1111/j.1474-9726.2006.00188.x
Gems, D., & McElwee, J. J. Ageing: Microarraying mortality. Nature 424, 259–261 (2003) (link to article)
Kenyon, C., et al. C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993) doi:10.1038/366461a0 (link to article)
Kimura, K. D., et al. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997) doi:10.1126/science.277.5328.942
Murphy, C. T., et al. Genes that act downstream of DAF-16 to influence the life span of Caenorhabditis elegans. Nature 424, 277–283 (2003) doi:10.1038/nature01789 (link to article)






























