« Prev Next »
Diabetes mellitus is a disease characterized by hyperglycemia (elevated glucose), which, because of its association with obesity, has become an epidemic in industrialized, first world societies. Physicians and researchers recognize that the defining characteristic of diabetes is low or absent insulin secretion and/or insulin signaling. Beyond insulin, the secretion of a second hormone, glucagon, may also be altered in diabetes. Given the central role of these hormones in diabetes, scientists are seeking to understand their role in the physiology and pathophysiology of the control of blood glucose. These hormones are produced within specialized "islands" of cells in the pancreas called islets (or islets of Langerhans). Although scientists have learned that glucose is the primary regulator of secretion in islets, a major focus of research in the field has been to define additional extracellular factors that regulate the secretion of both hormones, such as the hormone receptors and the intracellular signaling pathways they activate. One particularly intriguing group of molecules involved in islet cell signaling is the G protein-coupled receptors (GPCRs). GPCRs are a major class of receptors mediating extracellular messages to intracellular signaling pathways in islets, and, more importantly, have the potential to be drug targets. Given that, researchers are working to define the role of GPCRs in normal pancreatic islet physiology as well as in the pathophysiology of diabetes (Winzell & Ahren 2007).
How is Blood Glucose Regulated?
What is Diabetes Mellitus?
Diabetes results from elevated blood glucose levels that stems from the decreased secretion and/or action of insulin. Two major forms of diabetes exist, type 1 and type 2. Type 1 diabetes is characterized by a complete lack of insulin that leads to an inability of cells to take up glucose. In this type of diabetes, the immune system specifically destroys insulin-secreting beta cells in the pancreatic islets — the body's own immune system attacks its own cells (a form of autoimmune disease). Without supplemental insulin treatment, type 1 diabetes is fatal. In contrast to type 1 diabetes, type 2 diabetes results from insulin resistance. Insulin resistance refers to impaired insulin signaling and action at the level of the cell, and the primary cause of insulin resistance is obesity. The normal response of the body to insulin resistance is to secrete more insulin to overcome the insulin resistance. However, in some individuals, pancreatic beta cells fail to secrete sufficient amounts of insulin to overcome the insulin resistance, and type 2 diabetes results. In addition to impaired insulin secretion, type 2 diabetic patients also have elevated levels of glucagon, which worsens hyperglycemia by increasing glucose production by the liver. Type 2 diabetes accounts for ~90 percent of all cases of diabetes. Once thought of as a form of diabetes limited to adults, type 2 diabetes is now also seen in children, as obesity becomes more common among young age groups in the United States. Given the long term health consequences of diabetes, and its increasing appearance among young people, understanding the pathophysiology of diabetes, and finding better way to treat it, has become a high priority for the US biomedical research agenda.
What Stimulates Pancreatic Hormone Secretion from Pancreatic Islets?
Given the critical role that pancreatic islet cells play in type 2 diabetes, scientists are working to better define normal islet biology in contrast to the abnormalities associated with diabetes. This field of research was catalyzed in the 1920s by researchers in Toronto, including Drs. Banting and Best, who identified insulin as the anti-diabetic factor produced by the pancreas. This discovery changed the once fatal diagnosis of type 1 diabetes to a manageable disease. The importance of this work is reflected by their being awarded the Nobel Prize in Medicine for this discovery. Similarly, in the 1920s Kimball and Murlin observed that the pancreas also produced a factor which raises glucose levels. A few years later this factor was identified as glucagon.
Since these pivotal discoveries, we now know that islets comprise the endocrine portion of the pancreas. Within islets, beta cells secrete insulin, and alpha cells secrete glucagon. Scientists have identified three additional cell types in islets, including cells that secrete the hormones somatostatin, ghrelin, and pancreatic polypeptide Y. They have also discovered that the secretion of each of these hormones depends on specific factors. For example, beta cells secrete insulin in response to increased blood glucose levels, and this stimulates cells to take up glucose, an essential step in energy homeostasis. In contrast, alpha cells secrete glucagon in response to decreased blood glucose levels, and this hormone binds to glucagon receptors in the liver. This binding stimulates breakdown of glycogen and the release of glucose into the circulation. Although glucose is the main mediator of glucagon secretion, scientists have learned that circulating factors like catecholamines (e.g., adrenaline/epinephrine) and amino acids (the building blocks of proteins) also stimulate glucagon release, while insulin and free fatty acids inhibit its release. Like glucagon, multiple factors similarly regulate insulin secretion such as amino acids, neurotransmitters and various hormones. At the interface between these circulating factors and insulin/glucagon secretion are GPCRs, which in islets mediate the effects of many of the circulating factors, such as glucagon-like peptide-1, free fatty acids, and catecholamines. With this knowledge, scientists are seeking to better understand the role of GPCRs in islet biology and hormone secretion, and how signaling in the cell through these receptors is disrupted in diabetes.
Receptor Signaling and the Concept of an Agonist
Over the last 50 years, scientists have learned that chemicals in the blood influence cells, by acting as ligands for cell receptors and stimulating them to secrete hormones. A well-understood example of this is the regulation of thyroid hormone synthesis by the thyroid gland via the release of a hormone from the pituitary gland. Cells within the pituitary synthesize and release a hormone, thyroid stimulating hormone (TSH), which binds to a receptor on the plasma membrane of thyroid cells. This stimulates the synthesis and release of thyroid hormone by cells in the thyroid gland. Like TSH, other ligands can bind to a specific receptor and stimulate cellular responses like TSH. These imitative ligands are referred to as agonists, and can be naturally occurring hormones, environmentally-originating chemicals or man-made chemicals like pharmaceutical drugs.
GPCR Receptor Structure and Classification
Scientists are especially intrigued by the GPCR family of receptors because, although ~1000 GPCRs have been identified, the function of many of these, and the ligands which activate them, are still unknown. However, each year, scientists are identifying the ligands for some of these orphan GPCRs (GPCRs without a known ligand). This not only adds to our understanding of biology and signaling pathways in cells, but, more importantly, it is leading to exciting new possibilities for targets for drug therapy. This is being accomplished by synthesizing drugs which have similar structures to natural ligands to generate new molecules capable of binding to GPCRs and stimulating a response in the cell (i.e., they act as agonists) or preventing the natural ligand/agonist from binding to the GPCR, thereby blocking the response (i.e., they act as antagonists). As approximately 30% of current pharmaceutical agents work through GPCRs, the role of GPCRs in islets is of great interest to the scientific community.
GPCR Signaling Mechanisms
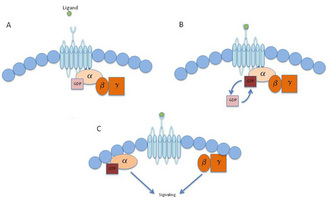
Research in GPCR biology is still quite young, and the importance of these receptors only grows as scientists learn more about their mechanisms of action. In the 1980s, Drs. Gilman and Rodbell (who jointly were awarded the Nobel Prize in 1994), established that molecules referred to as guanine nucleotide-binding proteins (G proteins) help send the signal from GPCRs located on the cell surface into the cells. Specifically, GPCRs associate with G proteins. G proteins are complexes of three protein subunits: the α, β, and γ subunits. These are called heterotrimers (hetero = different, tri = three) since they are made up of three different subunits (α, β, and γ). When the Gα subunit binds GDP (guanine diphosphate), it is in an inactive form. Upon activation, the Gα subunit releases GDP and binds to GTP (guanine triphosphate — the same molecule used to make DNA) to activate the subunit. The Gα subunit then releases the βγ complex, and the Gα subunit and the βγ complex each activate a variety of effector molecules. Each GPCR complexes with unique Gα subunits from one of the four major classes of α subunits (Gs, Gi, Gq, and G12/13), and each class consists of multiple subtypes. The sequencing of the human genome provided the knowledge that there are genes for 16 a subunits. Some of the genes encoding the a subunits can exist as multiple isoforms because of alternative splicing of mRNA, and scientists currently believe that there are a total of 23 different isoforms of the a subunit. In addition to multiple a subunits, 5 β subunits and 12 γ subunits were identified in the human genome, which allows for multiple combinations of βγ complexes. These pathways are also regulated by many proteins that can influence each step in the signal transduction pathway, allowing GPCRs to activate a broad range of pathways.
How Can a Better Understanding of GPCRs Lead to New Treatments for Diabetes?
Researchers are seeking to understand which (as well as how) GPCRs are involved in normal and diabetic islet function, as this knowledge has the potential to suggest new approaches to treat diabetes. Given the importance of GPCRs in transmitting signals from the extracellular environment and potential as drug targets, scientists have sought to understand their role in pancreatic islet cell biology. A first step in this process was to understand which GPCRs are expressed in islets. Shaun Coughlin and colleagues recently reported that mouse islets express high levels of at least 28 different GPCRs (Regard et al. 2007). Table 1 lists some of the GPCRs expressed by beta cells that are known to affect insulin secretion and their natural ligands. As the ligands and function of additional GPCRs are defined, more GPCRs that influence insulin secretion will likely be identified.
| Table 1 | |||||
| GPCRs | Full name | Ligand | Alpha subunit | Cell type | Insulin secretion |
| ADRB2 | Beta-2 adrenergic | Epinepherine | Gs | beta | + |
| ADRA2A | Alpha-2 adrenergic (A) | Norepinepherine | Gi | beta | - |
| MTNR1A | Melatonin 1A | Melatonin | Gq/Gi | beta | ? |
| MTNR1B | Melatonin 1B | Melatonin | Gq/Gi | beta | ? |
| HTR2B | Serotonin-2B | Serotonin | Gq | beta | + |
| HTR1D | Serotonin-1D | Serotonin | Gi | beta | - |
| M3 | Muscarinic-3 | Acetylcholine | Gq | beta | + |
| SSTR2 | Somatostatin-2 | Somatostatin | Gi | beta | - |
| GLP1R | Glucagon-like Peptide 1 | Glucagon-like Peptide 1 | Gs | beta/alpha? | + |
| GPR40 | G-protein receptor40 | Free fatty acids | Gq | beta | + |
| GPR119 | G-protein receptor119 | Free fatty acids | Gs | beta | + |
| GCGR | Glucagon | Glucagon | Gs/q | beta/alpa | + |
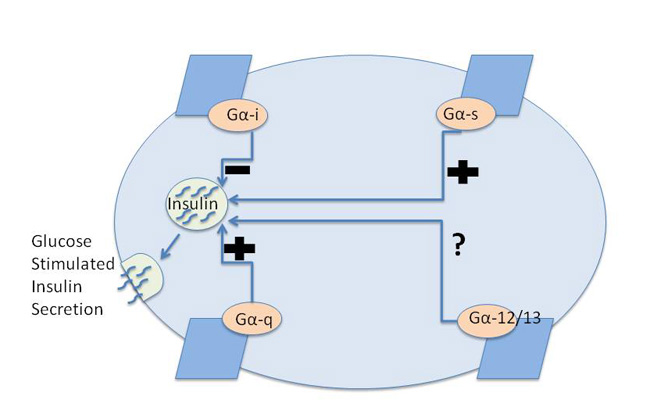
Although glucose levels are a primary regulator of insulin secretion, signaling through different GPCRs can have positive or negative effects on insulin secretion through their regulation of intracellular signaling pathways. Identifying the downstream signaling partners for GPCRs has improved our understanding of this regulation of insulin secretion. There are some generalizations that can be made about the effects of GPCRs on insulin secretion based on their Gα subunit coupling preferences (Figure 3). GPCRs that signal through the Gαq and Gαs pathways tend to increase insulin secretion, whereas GPCRs that signal through the Gαi pathway generally inhibit insulin secretion. It is important to remember, however, that GPCRs couple to a complex of Gαβγ, so activation of GPCRs results in the release of Gβγ as well as Gα subunits. One of the unanswered questions is what role, if any, different Gβγ complexes play in insulin secretion. What has also become apparent to investigators in the field is that individual GPCRs often interact with more than one type of Gα subunit. Thus, the effects of any one type of GPCR on insulin secretion may be quite complex.
One group of GPCRs present in islets is the adrenergic family of receptors. These receptors are named for their agonists, adrenalin (epinephrine) and noradrenalin (norepinehrine), examples of catecholamines. While these receptors play a key role in the regulation of blood pressure, scientists now know that members of the adrenergic family of GPCRs, including both the α2 and β2 receptors, also regulate islet function. For example, α2-adrenergic receptors inhibit insulin secretion and stimulate glucagon secretion, while β2-adrenergic receptors stimulate both insulin and glucagon secretion. Understanding the role of these receptors in islet function through cell biology and clinical studies has been complemented by recent genetic studies. These studies have identified one of these receptors (the α2 receptor) as being associated with the risk for type 2 diabetes. More specifically, what has been learned is that people who carry a specific mutation (single nucleotide polymorphism) in the gene encoding the α2 receptor (subtype 2A) have an increased risk of type 2 diabetes. This genetic variant is associated with overexpression of the α2 receptor and decreased insulin secretion (Rosengren et al. 2010).
The Role of GPCRs in Circadian Rhythms
New observations have especially intrigued scientists in light of the recently recognized association of sleep disturbances with obesity and risk for type 2 diabetes. Genetic studies have found that a GPCR well known to be involved in sleep rhythms is also important for islet cell function. This GPCR binds to melatonin. Melatonin is best known for its role in sleep and circadian rhythms, but researchers recently learned that individuals who carry a particular mutation in one of the GPCRs that bind to melatonin, melatonin receptor 1B (MTNR1B), have higher fasting glucose levels. With that new knowledge in hand, clinical investigators went on to discover that individuals with a mutation in the MTNR1B gene have impaired insulin secretion from their beta cells when glucose levels increase. One manifestation of this is decreased insulin secretion in response to the intake of glucose (Langenberg et al. 2009). Human islets express two different melatonin receptors, 1A and 1B, and the recent recognition of their potential importance to metabolism has increased interest in the role of these GPCRs in alpha and beta cells. While much remains to be learned in this new area of research, it is now clear that circadian rhythms can impact glucose metabolism, in part, through GPCRs expressed in islets.
Glucagon and Alpha Cells
To date, the characterization of GPCRs in islets has focused largely on beta cells, so less is known about the role of GPCRs in alpha cells. Interestingly, alpha cells not only secrete glucagon, but they also express the glucagon receptor, which is itself a GPCR. Thus, glucagon is both secreted by and acts on alpha cells to regulate their own secretion. Along with the glucagon receptor, it is likely that alpha cells express additional GPCRs that play roles in glucagon secretion. Researchers are actively seeking to characterize these receptors, because GPCRs that regulate glucagon secretion may be excellent targets for diabetes therapies due to their overall importance in the regulation of islet function.
GPCRs and Insulin Changes During Pregnancy
A variety of genetic studies suggested that another class of GPCRs plays an important role in islet function. Pregnancy is characterized by an increase in insulin resistance; specifically, the effect of insulin at the level of the cell is decreased. Because of this, scientists reasoned that pregnancy would serve as a good model for understanding how beta cells adapt to insulin resistance. During pregnancy, islets respond by secreting more insulin to overcome this resistance. This is accomplished largely via an increase in the size and number of beta cells in the pancreas. As type 2 diabetes is a disorder in which islets do not fully adapt to insulin resistance, the implications of these studies may be applicable to understanding the pathophysiology of type 2 diabetes. To address this question, Kim et al. (2010) compared the level of expression of genes in islets from pregnant and nonpregnant female mice. These studies identified serotonin and its receptors as being important in regulating islet function during pregnancy. More specifically, these researchers observed that pancreatic beta cells express higher levels of serotonin receptors 2b and 1d during pregnancy, and signaling through these receptors mediates, in part, the pregnancy-induced increase in the number of pancreatic beta cells.
How Has Defining the Role of GPCRs in Islet function Translated into Drug Discovery?
This topic has been reviewed in detail by Ahren and colleagues (2009), but one example is the success that has been achieved in the treatment of type 2 diabetes by targeting the glucagon like peptide-1 receptor (GLP1-R), which is a GPCR. In the 1970s and 80s, scientists defined a new set of molecules, incretins, which stimulate glucose-dependent insulin secretion when released by cells in the gut following a meal. Incretins include GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). When secreted by cells in the gastrointestinal tract, GLP-1 acts through its receptor on pancreatic islet cells to stimulate insulin secretion and inhibit glucagon secretion. Knowing that, targeting the GLP-1 receptor (GLP-1R) has been a major initiative in drug development research. New drugs capable of binding to the GLP-1R are now approved as a treatment for type 2 diabetes. These drugs not only enhance insulin secretion and inhibit glucagon secretion, but clinical researchers also noted that treatment with a GLP-1R agonist decreased appetite and induced early satiety, which resulted in significant weight loss in many patients. It is speculated that the effect on appetite is mediated through receptors in the brain.
Additional strategies have been used to increase GLP-1R activity, as researchers successfully developed drugs that inhibit the enzyme dipeptidyl peptidase IV (DPP-IV). DPP-IV is an enzyme which inactivates GLP-1, and inhibitors of the enzyme DPP-IV increase GLP-1 levels and, thus, prolong its effect. DPP-IV inhibitors are now available as another new treatment for type 2 diabetes. With the success in targeting GLP-1R for the treatment of type 2 diabetes, researchers and pharmaceutical companies continue to target GPCRs for drug development to treat type 2 diabetes.
What are some of the other potential targets? One family of GPCRs researchers are studying is a group of receptors activated by fatty acids. Along with glucose, free fatty acids present in the circulation are known to regulate insulin secretion. Recently, researchers showed that activation of GPCRs by fatty acids contributes to this effect. Four different GPCRs that bind fatty acids have been characterized, and two of these, GPR40 and GPR119, decrease glucose-stimulated insulin secretion (Kebede et al. 2009). This finding has heightened interest in these receptors as potential drug targets.
Summary
Glucose is one of the primary energy sources for the body, hence its metabolism is tightly controlled. Insulin and glucagon are key regulators of glucose levels in the circulation. Although both are produced by cells in pancreatic islets, insulin's primary function is to stimulate cells to take up glucose, whereas glucagon's primary function is to raise glucose levels when they fall. With disturbances in the secretion of these hormones, alterations in the level of glucose can occur. Diabetes, a major disease in US society, is characterized by elevated glucose levels. Although glucose itself is a primary regulator of the secretion of insulin and glucagon, additional factors regulate islet function and, thus, the secretion of insulin and glucagon. Many of these factors impact insulin and glucagon secretion by binding to GPCRs on the surface of beta and alpha cells. While much is known about GPCRs in islets, many areas await further study, such as the function of novel GPCRs without a known function that are expressed by beta cells, as well as the role of GPCRs in alpha cells. It is clear that increasing our understanding of the role of GPCRs and their signaling pathways in islets will enhance our knowledge of normal and abnormal metabolism. Moreover, new advances in the field will likely lead to additional GPCRs becoming effective drug targets in the future, providing novel approaches to the treatment of diabetes.
References and Recommended Reading
Ahrén, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature Reviews Drug Discovery 8, 369–385 (2009). doi:10.1038/nrd2782.
Kebede, M. A., Alquier, T., et al. Lipid receptors and islet function: therapeutic implications? Diabetes, Obesity and Metabolism 11, 10–20 (2009). doi: 10.1111/j.1463-1326.2009.01114.x.
Kim, H., Toyofuku, Y., et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature Medicine 16, 804–808 (2010). doi:10.1038/nm.2173.
Langenberg, C., Pascoe, L., et al. Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia 52, 1537–1542 (2009). doi: 10.1007/s00125-009-1392-x.
Regard, J. B., Kataoka, H., et al. Probing cell type-specific functions of G(i) in vivo identifies GPCR regulators of insulin secretion. Journal of Clinical Investigation 117, 4034–4043 (2007). doi:10.1172/JCI32994.
Rosengren, A. H., Jokubka, R., et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 327, 217–220 (2010). doi: 10.1126/science.1176827.
Winzell, M. S. & Ahren, B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacology and Theraputics 116, 437–448 (2007). doi:10.1016/j.pharmthera.2007.08.002.



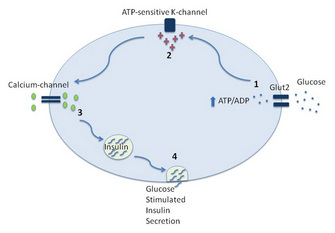
 Figure 1: The key steps leading to glucose-stimulated insulin secretion
Figure 1: The key steps leading to glucose-stimulated insulin secretion