« Prev Next »

Future Dengue Fever Treatments
Introduction
What Are Vaccines?
Producing Dengue Vaccines
Scientists have been attempting to produce a safe, effective dengue vaccine since the 1940s. Why is it so difficult to develop a suitable dengue vaccine? One challenge is that there are four distinct types (serotypes) of dengue virus that cause dengue, and an effective vaccine must successfully protect people against all four virus serotypes. Once a person has immunity against one dengue serotype, the individual will never be infected with that same serotype again. If the person is subsequently infected with a different dengue serotype, however, he or she will have an increased risk of developing a more severe dengue illness. Therefore, a safe vaccine must provide immunity against all four dengue viruses to protect people from severe dengue successfully.
Despite these concerns, scientists have made considerable progress in developing dengue vaccines. Scientists are testing candidate vaccines for safety and effectiveness and to ensure that they do not have negative side effects. Currently, five types of dengue vaccines are in development: live attenuated vaccines, chimeric live attenuated vaccines, inactivated vaccines, subunit vaccines, and nucleic acid-based vaccines. What are these vaccine types, and what are the differences between them?
Live Attenuated Vaccines
Live attenuated vaccines are made of weakened versions of the dengue virus (Figure 1A). An ideal live attenuated vaccine would produce a robust immune response similar to that of natural infection — but without the disease or symptoms of natural infection. Because the weakened virus does not replicate well, an ideal live attenuated vaccine would produce low levels of the virus in the blood and only minimal symptoms of infection. The low levels of virus in the blood would make it unlikely that mosquitoes could transmit the attenuated virus. Live attenuated vaccines are generally inexpensive to produce, which makes them widely available. In addition, because there are four dengue viruses, the ideal vaccine should provide balanced immunity to all four dengue serotypes.
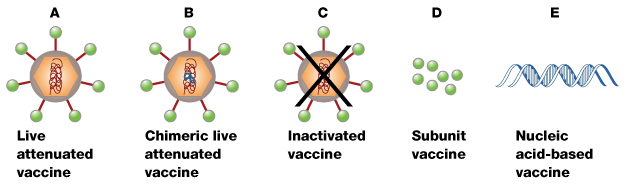
Chimeric Live Attenuated Vaccines
Inactivated and Subunit Vaccines
Nucleic Acid-based Vaccines
What are the characteristics of the nucleic acid-based vaccines? Nucleic acid-based vaccines are designed by introducing DNA copies of specific dengue viral genes into cells (Figure 1E). The dengue genes are expressed as dengue proteins, which produce an immune response. This type of vaccine is relatively simple to produce, but may require multiple doses to provide immunity. Therefore, this option may not be practical for widespread vaccinations.
Researchers continue to make progress toward developing safe, effective vaccines against dengue. In preclinical and clinical trials, a large number of vaccine candidates are currently being evaluated for their safety and effectiveness. It is possible that a safe and economical dengue vaccine will be commercially available in the next few years.
Dengue Antiviral Drugs
In addition to developing dengue vaccines, scientists are working to design antiviral drugs against dengue. Antiviral drugs are an alternative to vaccinations, but research on antivirals is a relatively new field. Once effective antiviral drugs are developed, they could potentially be administered to people with or without a fever to decrease or prevent disease symptoms at the first sign of a dengue outbreak.
Antiviral drugs work by reducing the level of virus in an infected person. Who would benefit from antiviral drugs? Patients who have very high levels of the dengue virus circulating in their blood have a greater risk of developing severe dengue disease than other patients. Scientists believe that reducing viral levels by administering antiviral drugs during the early stage of the dengue infection could decrease the risk of severe dengue diseases.
How are antiviral drugs developed? The development of antiviral drugs involves screening libraries of thousands of potential antiviral compounds to see if they can interfere with the dengue viral proteins necessary for viral replication. One example is using an antiviral drug called a nucleoside analogue to block a dengue infection. This compound works by preventing synthesis of the viral RNA genome so that the dengue virus cannot replicate. Scientists are currently testing and identifying nucleoside analogues to find those that can inhibit dengue but have no adverse side effects or toxicity.
As scientists have learned about the structures and functions of various viral proteins, they have been able to consider new targets for antiviral drugs. Research on the disease pathway of dengue infections can give scientists new avenues to develop dengue therapies. Viral replication can be difficult to prevent because there is a short timeline for treatment. Instead, scientists may also be able to target other steps in the disease pathway to prevent dengue at later stages. Once scientists develop safe antiviral therapies, they can administer them to people with or without a fever during dengue epidemics to decrease dengue symptoms.
Summary
References
Callaway, E. Dengue fever climbs the social ladder. Nature 448, 734–735 (2007) doi:10.1038/448734a
Cassetti, M. C. et al. Report of an NIAID workshop on dengue animal models. Vaccine 28, 4229–4234 (2010). doi:10.1016/j.vaccine.2010.04.045
Clarke, T. Dengue virus: Break-bone fever. Nature 416, 672–674 (2002). doi:10.1038/416672a
Crunkhorn, S. Adenosine analogue blocks dengue infection. Nature Reviews Drug Discovery 9, 21 (2010). doi:10.1038/nrd3081
Gubler, D. J. Dengue and dengue hemorrhagic fever. Clinical Microbiology Reviews 11, 480–496 (1998).
Mahoney, R. et al. Dengue vaccines regulatory pathways: A report on two meetings with regulators of developing countries. PLoS Medicine 8, e1000418 (2011). doi:10.1371/journal.pmed.1000418
Noble, C. G. et al. Strategies for development of dengue virus inhibitors. Antiviral Research 85, 450–462 (2010) doi:10.1016/j.antiviral.2009.12.011
Rothman, A. L. Dengue: Defining protective versus pathologic immunity. The Journal of Clinical Investigation 113, 946–951 (2004). doi:10.1172/JCI21512
Stephenson, J. R. Understanding dengue pathogenesis: Implications for vaccine design. Bulletin of the World Health Organization 83, 308–314 (2005) doi:10.1590/S0042-96862005000400016
Whitehead, S. S. et al. Prospects for a dengue virus vaccine. Nature Reviews Microbiology 5, 518–528 (2007). doi:10.1038/nrmicro1690
Wilder-Smith, A. et al. Update on dengue: Epidemiology, virus evolution, antiviral drugs, and vaccine development. Current Infectious Disease Reports 12, 157–164 (2010). doi:10.1007/s11908-010-0102-7
World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization and the Special Programme for Research and Training in Tropical Diseases, 2009.
World Health Organization, Initiative for Vaccine Research. "Vector-Borne Viral Infections" (2011).
Yin, Z. et al. An adenosine nucleoside inhibitor of dengue virus. Proceedings of the National Academy of Sciences 106, 20435–20439 (2009). doi:10.1073/pnas.0907010106.





















