« Prev Next »
People receive environmental cues through their five senses, but how do genes sense the environment? In the case of human health, it is clear that a person's environment can affect disease risk and development, which suggests that environmental cues must have some way of reaching cellular nuclei and orchestrating changes in genes or gene usage. Research indicates that these effects can be achieved through a number of mechanisms. In some cases, such as cell signaling, cells have biochemical machinery that is used to transmit information from the outside in. In other cases, sufficiently small or powerful environmental cues, such as radiation, can directly exert their impact upon a cell's DNA.
Cell Signaling

One example of cell signaling involves the migration of single-celled amoebae of the slime mold Dictyostelium into a single, cooperative fruiting body. Specifically, these amoebae secrete certain molecules that organize their movement, causing them to line up and march like soldiers to a single, central destination (Figure 1; Kessin, 2003). This process, known as chemotaxis or chemically induced movement, is mediated by a ligand called cyclic AMP (cAMP) (Konijn et al., 1969). Receptors for cAMP are found on the surface of each amoeba; the binding of these receptors with the secreted cAMP triggers a chain of events that is known generically as a signal cascade. During this cascade, receptor-ligand binding leads to molecular changes in the receptor that are, in turn, passed along to other intracellular proteins. Subsequently, as each protein acquires a change, it causes a change in the next protein in the cascade, ultimately leading to changes in gene transcription within the nucleus.
Inside the nucleus, cell signaling can cause alterations in gene expression by influencing the availability or activity of transcription factors. Transcription factors are proteins that bind near transcription start sites, thereby guiding the RNA transcription complex and activating or repressing transcription. In this way, transcription factors are essential elements for mediating changes in gene usage. Thus, you can imagine how a trail of attractive cAMP brings about a change in cellular behavior, such as the urge to march. In this case, cAMP-mediated changes in gene expression affect the genes involved in cell motility.
Although this example is visually stunning, the overriding theme is actually quite common in biology. In fact, small-molecule signaling is the process behind a large number of common biological events. Examples include phototransduction (in which photon reception leads to a series of chemical changes in response to visual cues), neurotransmitter signaling between neurons, immune cell homing to wound sites, and many other processes that occur during development and adulthood.
Mutagens: A Direct Route into the Nucleus
Although small molecules can lead to phenotypic changes via cell signaling, other environmental agents find more direct routes into a cell's nucleus. These agents are known as mutagens, and they act by directly altering a cell's DNA sequence. Many mutagens, by virtue of their size or structure, can slip through both cell and nuclear membranes and interact with DNA directly, usually resulting in damage. Some examples of these substances include chemical mutagens and radiation, both of which result in DNA damage, albeit for different reasons.
Chemical Mutagens
Chemical mutagens alter the delicate chemistry of the base pairs that compose the DNA chain through a variety of mechanisms. Some mutagens strip DNA nucleotides (bases) of essential modifications—for example, they deaminate the bases—such that these bases resemble different nucleotides and confuse the DNA replication machinery. Subsequent rounds of DNA replication then permanently incorporate such changes. Other mutagens, such as ethidium bromide, a common lab reagent, look so much like base pairs that they intercalate (or stick) between nucleotides, which can lead to insertion or deletion of an extra base pair following the next round of DNA replication.
Radiation
Radiation is another type of environmental mutagen that may cause direct changes in a cell's DNA. For instance, ionizing radiation (i.e., X-rays) can break DNA sequences in many places, leading to chromosome rearrangement. Lower-energy radiation, such as UV rays, can also penetrate cellular and nuclear membranes. One way this penetration can damage DNA is by cross-linking (chemically gluing) two bases together. In addition, both ionizing and UV radiation can cause double-stranded DNA breaks. Cells generally attempt to fix such breaks by a process called homologous recombination, but sometimes this process goes wrong. For example, when multiple breaks are joined together in the wrong order (a phenomenon known as nonhomologous end-joining), the result can be a loss of large, gene-rich regions of DNA. Because accurate repair of such damage is so important, cells have cell cycle checkpoints, or mechanisms designed to stall continued cell division until the DNA can be repaired.
Mutations and Cancer
Interestingly, DNA repair pathway genes are often mutated in cancers and other genetic disorders. For instance, xeroderma pigmentosum, a disorder of the skin, is caused by mutations in DNA repair enzyme genes, and these mutations can lead to greatly increased susceptibility to skin cancer.
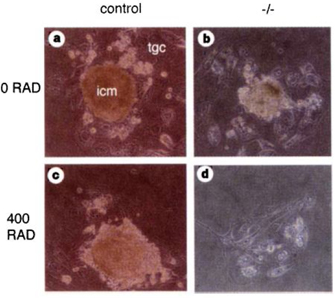
Genetic deficiencies can also compound susceptibility to breast cancer (Ronckers et al., 2005). For example, mutations in two essential cell cycle regulator genes, BRCA1 and BRCA2, have been found to be associated with breast cancer, raising the possibility of a failure in DNA repair. Early evidence that suggested these genes were involved in DNA repair was mostly circumstantial. For instance, the expression levels of these genes correlate with cell division, peaking during the S phase, therefore suggesting a role in replication, which is also when DNA repair occurs (Blackshear et al., 1998). The fact that the BRCA proteins also interact directly with a known DNA repair protein, Rad51, was also suggestive that the BRCA proteins are involved in correcting DNA damage (Scully et al., 1997). Direct evidence for this supposition finally came when scientists examined cells with defective BRCA1 and BRCA2 genes to determine how they responded to an agent known to cause DNA damage: radiation (Xu et al., 1999; Sharan et al., 1997). Sharan's research team in particular demonstrated that cells lacking a wild-type BRCA2 gene were hypersensitive to DNA damaging agents. In this case, cells from either wild-type (having functional BRCA2) or "null" (having no functional BRCA2) mice were exposed to radiation. The null cells were unable to survive the damage, whereas the normal cells survived, presumably because they retained the capacity to repair radiation-related damage (Figure 2).
Evidence also exists that the BRCA proteins play a role in homologous recombination (Powell & Kachnic, 2003). Specifically, cells that lack a normal BRCA2 gene exhibit up to a hundredfold reduction in the rate of homologous recombination (Moynahan et al., 2001). Thus, mutations in the BRCA genes have been proposed to allow misregulation of DNA repair possibly leading to tumorigenesis, although other mechanisms have also been proposed.
How Big of a Problem Are Mutations?
Changes in DNA caused by mutagens can have a range of effects, depending on where these changes happen to occur within the genome. Some mutations are silent, exerting no effect upon the amount or quality of the protein made from a given gene. Other mutations can be more severe, and they may even lead to complete loss of protein production. Fortunately, humans have two active copies of most genes, which provides some protection against the deleterious effects of mutation. However, this is not always the case. For instance, males have only one copy of the genes carried on the X chromosome, and an entire copy of this chromosome is silenced in females. Moreover, gene-gene interactions, or epistasis, can amplify the consequences of partial loss of a single gene. That is, if multiple genes are required for a particular process, damage to even a single copy of one of those genes can damage the entire pathway. The role of gene interactions in the etiology of human disease is therefore a critical area of study.
References and Recommended Reading
Blackshear, P. E., et al. BRCA1 and BRCA2 expression patterns in mitotic and meiotic cells of mice. Oncogene 16, 61–68 (1998)
Connor, F., et al. Tumorigenesis and a DNA repair defect in mice with a truncating BRCA2 mutation. Nature Genetics 17, 423–430 (1997) (link to article)
Kessin, R. H. Cell motility: Making streams. Nature 422, 481–482 (2003) (link to article)
Konijn, T. M., et al. Identification of adenosine-3', 5'-monophosphate as the bacterial attractant for myxamoebae of Dictyostelium discoideum. Journal of Bacteriology 99, 510–512 (1969)
Moynahan, M. E., et al. BRCA2 is required for homology-directed repair of chromosomal breaks. Molecular Cell 7, 263–272 (2001)
Powell, S. N., & Kachnic, L. A. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene 22, 5784–5791 (2003)
Ronckers, C. M., et al. Radiation and breast cancer: A review of current evidence. Breast Cancer Research 7, 21–32 (2005)
Scully, R., et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88, 265–275 (1997)
Sharan, S. K., et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking BRCA2. Nature 386, 804–810 (1997) (link to article)
Wong, A. K. C., et al. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene BRCA2. Journal of Biological Chemistry 272, 31941–31944 (1997)
Xu, X., et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Molecular Cell 3, 389–395 (1999)



 Figure 1: A gathering of Dictyostelium discoideum amoebae
Figure 1: A gathering of Dictyostelium discoideum amoebae


























