« Prev Next »
To be able to map the location of disease-associated genes within a genome, scientists first needed to determine the landscape of human chromosomes. How many chromosomes are present in a human cell? Are all human chromosomes the same size? How can we tell them apart? We now know the answers to these questions, but the first of these discoveries wasn't made until 1956, when Tjio and Levan provided the first accurate count of the number of chromosomes in a human cell: 46. This knowledge led researchers in new directions that facilitated gene mapping and discovery. In fact, one of the earliest methods used to study human chromosomes - Giemsa staining, which yields signature banding patterns for each of the 24 types of human chromosomes - is still in use today. By combining Giemsa staining with somatic cell hybrids, which contain a mixture of human and mouse chromosomes, scientists were eventually able to map disease-associated genes to individual chromosomes.
Human Chromosome Anatomy
Human chromosomes come in 24 varieties: 22 different autosomes and two different sex chromosomes (X and Y). Moreover, somatic cells contain 23 pairs of chromosomes, including 22 autosomal pairs and one pair of sex chromosomes. Before various staining techniques were developed, researchers were only able to distinguish these different types of chromosomes from each other based on their length and the position of their centromere, which is the "waistline" of the chromosome where the two sister chromatids are attached following DNA replication. Researchers were also aided in this endeavor by the fact that human chromosomes are not symmetrical, and the two arms that extend from the centromere are often different lengths. The short arm of a human chromosome is referred to as the p arm, while the long arm is called the q arm. The ends of chromosomes are called telomeres.
G-Bands Distinguish Individual Human Chromosomes
In 1971, researchers Maximo Drets and Margery Shaw developed a method for staining human chromosomes using Giemsa dye that led to distinct banding patterns (called G-bands) for each of the 24 types of human chromosomes (Drets & Shaw, 1971). In their experiments, Drets and Shaw took cultures of human lymphocytes and fibroblasts that were growing and undergoing mitosis and exposed them to a drug called colchicine, which disrupts microtubules and causes cells to arrest in metaphase with duplicated chromosomes consisting of two sister chromatids. The cells were then exposed to hypotonic conditions to permeabilize them. After that, the cells were fixed in a mixture of methanol and acetic acid, which allowed them to be flame dried and adhered to a glass microscope slide. Next, the chromosomes from the cells were treated with sodium hydroxide and increasing concentrations of sodium citrate, after which Giemsa staining yielded distinct and reproducible G-banding patterns.
Drets and Shaw then photographed and generated G-banding maps for each human chromosome. They found that each pair of chromosomes within a cell showed a similar G-banding pattern (Figure 1). Furthermore, they found that the G-banding patterns were the same for a given chromosome when they compared different cells.
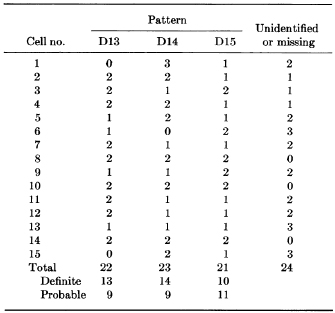
Drets and Shaw also carried out a "blind" experiment in which they analyzed the G-banding patterns in 15 cells from three males (Drets & Shaw, 1971). Here, the duo photographed the chromosomes in each of the 15 cells, karyotyped them by cutting out individual photographs of each chromosome, and labeled the back of the photos to designate which cell each chromosome came from. Next, they put the chromosomes into groups according to their length and centromere position without looking at the banding patterns. One group of three chromosomes, called group D, was examined in more detail. Drets and Shaw wanted to determine whether the number of types of G-banding patterns was the same as the number of group D chromosomes, and whether each cell contained two chromosomes with each G-banding pattern.
Drets and Shaw then examined the G-banding patterns of three pairs of group D chromosomes from each of the 15 cells in order to identify similar patterns in each of the chromosomes. Immediately, they noted three distinct types of G-banding patterns, which they termed D13, D14, and D15. The duo then determined the G-banding patterns within each of the 15 cells, and found that each cell contained two copies apiece of the D13, D14, and D15 chromosomes. As shown in Figure 2, each of the 15 cells examined (numbered 1-15) typically showed two chromosomes with each of the three group D G-banding patterns (D13, D14, and D15). Sometimes, however, not all of the group D chromosomes could be identified, or they were not present in the photograph, a fact that highlights the real-world difficulties associated with studying chromosomes.
Collectively, the results of these experiments demonstrate that each autosome and sex chromosome can be clearly distinguished when its length, centromere position, and G-banding pattern are taken into account. Furthermore, researchers found that banding techniques made it possible to determine which chromosomes were involved in various types of structural rearrangements. Indeed, the ability to determine the nature of chromosomal translocations and deletions contributed immensely to the discovery and mapping of human disease-associated genes. By determining which part of a chromosome was deleted or translocated, scientists could determine the approximate chromosomal location of a disease-associated gene. For example, the RB1 tumor suppressor gene was mapped to the X chromosome based on the ability to visualize a deleted chromosomal region in cells from individuals with retinoblastoma and a loss of chromosome band 13q14 (Francke & Kung, 1976; Knudson et al., 1976).
Human-Mouse Somatic Cell Hybrids
Also in 1971, researchers Jacques Jami and Simone Grandchamp carried out a series of experiments in which they used the Sendai virus to induce the fusion of mouse and human somatic cells to generate a human-mouse somatic cell hybrid (Jami & Grandchamp, 1971). They found that the resulting hybrid cells rapidly lost many of the human chromosomes but retained the mouse chromosomes. Indeed, after 20 cell divisions, the hybrid cells retained only between 1 and 15 of the original human chromosomes. Occasionally, the hybrid cells contained two complete sets of mouse chromosomes; in this case, the human chromosomes were retained at higher levels, as shown by a persistence of 10 or more human chromosomes after 150 cell divisions. In the years that followed, various researchers were able to generate a series of human-mouse somatic cell hybrid lines that stably maintained a small, defined number of human chromosomes.
At first glance, these efforts to create human-mouse somatic cell hybrids may seem more like science fiction than true science. However, being able to generate cells that contain small numbers of human chromosomes and then use G-band staining patterns to determine precisely which human chromosomes are present greatly contributed to our ability to map and clone human genes. For instance, human-mouse somatic cell hybrids played a key role in the mapping and eventual cloning of the Huntington's disease-associated gene, HTT. Indeed, researchers found that if they could identify a DNA probe linked to a human disease, they could use this probe in Southern blot experiments with chromosomal DNA from a series of well-defined human-mouse somatic cell hybrids. They could then determine which human chromosome was common among those hybrid cell lines to which the disease-associated DNA probe could bind. Using this method, researchers were able to map 1,148 human genes by 1991 (McKusick, 1991).
Chromosomes Are a Useful Tool for Mapping Disease Genes
Thanks to the completion of the Human Genome Project, and due to our knowledge of chromosomes at the base-pair level, somatic cell hybrids are no longer the first option for mapping genes. However, the ability to identify chromosomes and the location of defects associated with them remains a valuable tool. Identifying a chromosomal breakpoint that is associated with a clinical manifestation (e.g., mental retardation or cancer) can often provide the location of the gene that is likely disrupted and causing the defect. Although these chromosomal defects are extremely rare, they are highly treasured among human genetics researchers. One such advance was the cytological identification and characterization of the Philadelphia chromosome, one of the most famous chromosomal translocations, which is associated with chronic myelogenous leukemia.
Through the study of rare cases involving chromosomal defects, a number of genes have been linked to disease phenotypes. For example, Felix Mitelman's Catalog of Chromosome Aberrations in Cancer, which was first published almost two decades ago, contains over 7,100 references encompassing some 100,000 aberrations related to cancer. In addition, in the case of the Philadelphia chromosome, cytological observations were met head-on by the development of one of the most highly praised cancer drugs of the past decade, Gleevec. Thus, even though techniques like G-banding provide data at relatively low resolution, a great deal of valuable information can be gleaned from these data with regard to human genetics, chromosomal biology, and the role of chromosome structure in human disease.
References and Recommended Reading
Drets, M. E., & Shaw, M. W. Specific banding
patterns of human chromosomes. Proceedings
of the National Academy of Sciences 68,
2073–2077 (1971)
Francke, U., & Kung, F. Sporadic bilateral retinoblastoma and 13q chromosomal deletion. Medical and Pediatric Oncology 2, 379–385 (1976)
Jami, J., & Grandchamp, S. Karyological properties of human-mouse somatic hybrids. Proceedings of the National Academy of Sciences 68, 3097–3101 (1971)
Knudson, A. G., Jr., et al. Chromosomal deletion and retinoblastoma. New England Journal of Medicine 295, 1120–1123 (1976)
McKusick, V. A. Current trends in mapping human
genes. FASEB Journal 5, 12–20 (1991) (link to article)
Tjio, J. H., & Levan, A. The chromosome numbers of man. Hereditas 42, 1–6 (1956)




 Figure 1
Figure 1