« Prev Next »
Knowledge about recessive X-linked gene-disease associations has led to the development and widespread use of prenatal diagnostic tests that can provide parents with information about whether their embryo or fetus might be carrying a disease mutation. Based on that information, the parents can either terminate the pregnancy or prepare for their future child, depending on their beliefs. You might expect that this kind of widespread testing has had the ultimate effect of decreasing the frequency of disease alleles in the population. In fact, the opposite is true, according to research by geneticist Ian Hastings of the Liverpool School of Tropical Medicine in England. To better understand Hastings's findings, let's consider them in the context of Duchenne muscular dystrophy (DMD).
Transmission, Causes, and Treatment of DMD
DMD is a fatal neuromuscular disorder caused by a recessive mutation on the X chromosome. Because the related mutation is recessive, DMD is more common in boys than in girls, as boys do not have another copy of the X chromosome to compensate for the genetic defect. DMD affects about 1 in 3,500 males. On the other hand, most girls born with DMD mutations are merely carriers, because they each possess only one mutated DMD gene on one of their two X chromosomes. Although not themselves affected by DMD, carriers can pass DMD genes on to their children.
Although DMD is named after the French neurologist Duchenne de Boulogne, who first described the disorder in the late 1800s, the cause of this disease remained a medical mystery until the mid-1980s, at which time the DMD gene defect was identified. It is now known that the DMD gene encodes dystrophin, a huge muscle protein with about 4,000 amino acids that plays an integral role in maintaining the structural integrity of muscle cells. Cells with mutated DMD genes cannot make normal dystrophin, which means that they are unable to function appropriately. The deleterious effect of this lack of dystrophin becomes apparent early in life, although usually not immediately. In fact, most babies with DMD appear normal at birth and don't start showing their first symptoms — muscle weakness — until between the ages of three and five years. Affected children then experience increasing difficulty in performing daily tasks, such as climbing stairs and playing with their peers. Indeed, most DMD patients rely on the use of a wheelchair by their early teens, with their muscles becoming progressively weaker and more atrophied (Figure 1). Patients usually die from heart or respiratory problems by their twenties or thirties, if not before.
Scientists have been searching for ways to cure DMD for as long as they have known about the disease. Today, based on the wealth of knowledge about the underlying genetic and molecular mechanisms of DMD, researchers are actively testing a variety of different therapeutic approaches (Khurana & Davies, 2003). These include gene-based therapies (e.g., replacing a patient's faulty DMD genes with normally functioning ones), cell-based therapies (e.g., replacing dystrophin-deficient muscle cells with stem cells from healthy donors), and drug-based therapies (e.g., using drugs to compensate in other ways for the dysfunctional dystrophin protein). Most of these initiatives have not provided any definitive answers. Indeed, few of the treatments currently being developed and tested hold forth promise that they will actually be used in the future. DMD thus remains a devastating disease for which there is no cure.
DMD Testing
Today, many parents who suspect that their embryo or fetus might have a mutated DMD allele or other X-linked recessive mutation rely on information gathered from different types of prenatal tests to make decisions about whether to terminate their pregnancy. For example, for DMD and some types of X-linked mental retardation with clearly identifiable disease-associated alleles, there are diagnostic tests available that can detect the presence of the disease-associated mutation in a fetus (Chelly & Mandel, 2001). For other X-linked recessive diseases for which we still do not know the causal genetic defect, parents instead rely on identification of the sex of the embryo (i.e., "embryo sexing"), aborting all males and carrying only females to term. The latter approach in particular touches on some sensitive ethical issues, because half of the discarded male embryos would not be affected and would presumably be healthy.
DMD Frequency: Hastings’s Findings
Prenatal diagnostic testing and embryo sexing for sex-linked recessive disease mutations bring up more than ethical issues, however. They also raise some interesting questions about how we as a society can affect the genetic structure of the human population at large, as Hastings (2001) emphasized in his study. Normally, a balance exists between mutation and selection. Deleterious mutations, such as sex-linked disease genes, disappear over time because affected individuals often die before they reach reproductive age or are unable to reproduce. In effect, these mutations are ousted from the gene pool by natural selection. Hastings argues that prenatal testing upsets this balance.
In the past, before prenatal testing or embryo sexing was an option, with no way to know whether a fetus had or might be carrying a deleterious sex-linked mutation, parents were not able to make these reproductive decisions. All fetuses affected with disease genes were born. Then, in the case of DMD, for example, if the affected child was a boy, he would mostly likely either die before reproducing or be incapable of reproducing, thereby removing that individual affected gene from the population. For this reason, diseases such as DMD have continued to occur at relatively low frequencies in the human population.
Hastings took a mathematical modeling approach to show how modern reproductive technologies have the opposite effect: They often result in an increased frequency of sex-linked, disease-causing mutations in a population. This is because, as Hastings argues in his paper, if a woman decides to terminate her pregnancy and then in the future tries to give birth to an unaffected child, a one-in-three chance exists that the next child will be a female carrier (meaning a daughter with one disease allele). So, instead of natural selection removing a mutation from the population, the population would actually gain a mutation. Over time, with many parents making this decision, the number of X-linked, disease-associated recessive mutations in the population would actually increase. In fact, Hastings calculated that the frequency of these mutations could increase as much as 33% or more, and in as quickly as two to five generations. Although easing their own family burden, parents could simultaneously contribute to an increased frequency of deleterious X-linked mutations in the population at large. It is debatable, however, whether this creates a problem for society, because even though the frequency of the lethal mutations would increase, the number of babies born with DMD would decrease.
In fact, based on the results of his mathematical simulations, Hastings argues that the only circumstance under which the number of babies born with lethal recessive X-linked mutations would actually increase, along with the frequency of the mutation itself, is when parents decide not to terminate a pregnancy, whether they have undergone prenatal testing or not, and instead practice another form of family planning. Specifically, parents who decide to let all pregnancies come to term and then, in the event of a baby being born with a fatal sex-linked disease, later "compensate" by having another child, contribute in the same way to the increasing population frequency of the disease allele; remember, there is a one-in-three chance that the next child will be a female carrier. By not terminating the pregnancy, the parents contribute to the number of babies being born with the disease.
Hastings's modeling results have yet to be verified with real data, so questions remain about whether recessive X-linked disease mutations are indeed increasing in frequency in populations in which these three reproductive technologies or behaviors (prenatal genetic testing, embryo sexing, or family planning) are being used on a widespread basis. Even then, questions would remain about whether the observed numbers were a direct or an indirect result of the widespread use of diagnostic tests; in other words, whether the diagnostic testing actually affects population structure, as Hastings predicts, or simply makes it easier to detect mutations that were previously undetectable (Casci, 2001).
References and Recommended Reading
Casci, T. Reproductive technologies: A long-term cost. Nature Reviews Genetics 2, 489 (2001) doi:10.1038/35080540 (link to article)
Chelly, J., & Mandel, J. L. Monogenic causes of X-linked mental retardation. Nature Reviews Genetics 2, 669–679 (2001) doi:10.1038/35088558 (link to article)
Hastings, I. M. Reproductive compensation and human genetic disease. Genetic Research 77, 277–283 (2001)
Khurana, T. S., & Davies, K. E. Pharmacological strategies for muscular dystrophy. Nature Reviews Drug Discovery 2, 379–390 (2003) doi: 10.1038/nrd1085 (link to article)



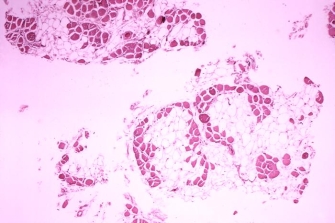
 Figure 1: Histopathology of Duchenne muscular dystrophy (DMD)
Figure 1: Histopathology of Duchenne muscular dystrophy (DMD)