« Prev Next »

What are Stable Isotopes?
Most elements exist in two or more forms, known as isotopes. Isotopes have the same number of protons but differ in their number of neutrons, resulting in different masses. The lighter form is generally the more common one (Hobson & Wassenaar 2008). This variation in the relative abundance of stable isotopes results from tiny mass differences that cause the isotopes to act differently in chemical reactions and physical processes. The lighter isotope generally forms weaker bonds than the heavier one and tends to react faster. The change in isotopic abundance is called fractionation (Karasov & Martínez del Rio 2007). Different environments are often characterized by predictable isotopic signatures (West et al. 2006).
Stable isotopes are measured as isotopic deviations from international standards and are expressed as delta (δ) values as parts per thousand (Werner & Brand 2001). These values are calculated as follows:
δ X = [(Rsample/Rstandard) – 1] x 1000
where X is the element (such as 13C or 15N), and R is the corresponding isotope ratio (13C/12C or 15N/14N). The quotient of the ratios in the sample relative to the standard is the δ value. The numerical values associated with the isotope ratio (such as δ12C) are the atomic masses of the isotopes and are accounted for by differences in the number of neutrons contained in the atom's nuclei. For example, δ12C contains 6 protons, 6 electrons and 6 neutrons, while its heavier isotope δ13C contains 6 protons, 6 electrons, and 7 neutrons. Therefore, an increase in the δ values denotes an increase in the amount of the heavier isotope component; while a decrease in the values denotes a decrease in the heavy isotope content (Peterson & Fry 1987).
'You Are What You Eat'
Stable isotope analysis is based on the principle ‘you are what you eat.' Stable isotope ratios vary among food webs and are incorporated into an animal's tissue via its diet (Hobson 1999). It is thus sometimes possible to infer the whereabouts of an animal moving between food webs. It is important, however, to choose the appropriate tissue for isotopic analysis, as tissues differ in how metabolically active they are (Rubenstein & Hobson 2004). Keratin-based tissues, such as hair, feather, nail, claw or bill, are metabolically inert after synthesis. They are usually used to study seasonal movements because an isotopic record reflecting the location of tissue synthesis remains unchanged. Conversely, metabolically active tissues provide dietary and source information for a relatively shorter period. For example, blood plasma and liver turnover elements in a matter of days or even hours, but this process takes up to several weeks in muscle and whole blood, and up to several months or even years in bone collagen (Hobson 1999). Studies that examine long-term movements therefore use metabolically inert tissues, while studies on recent movements (e.g., distinguishing between newly arrived and resident individuals) use metabolically active tissues with rapid turnover rates.Stable Isotopes Used in Terrestrial Systems
Carbon (13C/12C)
Nitrogen (15N/14N)
Hydrogen (2H/1H)
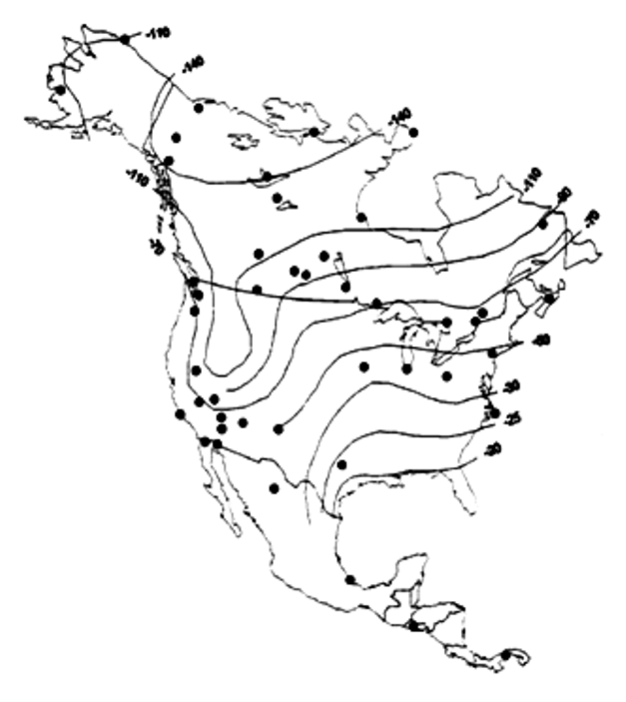
Stable Isotopes in Aquatic Systems
Carbon (13C/12C)
Nitrogen (15N/14N)
Oxygen (18O/16O)
Strontium (87Sr/86Sr)
Sulfur (34S/32S)
References and Recommended Reading
Alisaukas, R. T. & Hobson, K. A. The determination of lesser snow goose diets and winter distribution using stable isotope analysis. Journal of Wildlife Management 57, 49-54 (1993).
Bearhop, S. et al. A forensic approach to understanding diet and habitat use from stable isotope analysis of (avian) claw material. Functional Ecology 17, 270-275 (2003).
Best, P. B. & Schell, D. M. Stable isotopes in southern right whale (Eubalaena australis) baleen as indicators of seasonal movements, feeding and growth. Marine Biology 124, 483-494 (1996).
Casey, A. L. & Meehan, T. D. Estimating the latitudinal origins of migratory birds using hydrogen and sulphur stable isotopes in feathers: Influence of marine prey base. Oecologia 134, 505-510 (2003).
Fleming, T. H. et al. Seasonal changes in the diets of migrant and non-migrant nectarivorous bats as revealed by carbon stable isotope analysis. Oecologia 94, 72-74 (1993).
Fry, B. Natural stable isotope tag traces Texas shrimp migrations. Fishery Bulletin 79, 337-345 (1981).
Godbout, L. et al. Sulfur isotopes in otoliths allow discrimination of anadromous and non-anadromous ecotypes of salmon (Oncorhynchus nerka). Environmental Biology of Fishes 89, 521-532 (2010).
Harrington, R. R. et al. N enrichment in agricultural catchments: Field patterns and applications to tracking Atlantic Salmon (Salmo salar). Chemical Geology 147, 281-294 (1998).
Hobson, K. A. Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 120, 314-326 (1999).
Hobson, K. A. & Wassenaar, L. I. Tracking Animal Migration with Stable Isotopes. London, UK: Academic Press, 2008.
Hobson, K. A. et al. Using stable isotopes to determine seabird trophic relationships. Journal of Ecology 63, 786-798 (1994).
Karasov, W. H. & Martinez del Rio, C. Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins. Princeton, NJ: Princeton University Press, 2007.
Kennedy, B. P. et al. Natural isotope marks in salmon. Nature 387, 776-767 (1997).
Killingley, J. S. Migrations of California gray whales tracked by oxygen-18 variation in their epizoic barnacles. Science 207, 759-760 (1980).
Killingley, J. S. & Lutcavage, M. Loggerhead turtle movements reconstructed from O and C profiles from commensal barnacle shells. Estuarine, Costal and Shelf Science 16, 345-349 (1983).
Marra, P. P., Hobson, K. A. & Holmes, R. T. Linking winter and summer events in a migratory bird using stable carbon isotopes. Science 282, 1884-1886 (1998).
Minami, H. & Ogi, H. Determination of the migratory dynamics of the sooty shearwater in the Pacific using stable carbon and nitrogen isotope analyses. Marine Ecology Progress Series 158, 249-246 (1997).
Mizutani, H. et al. Carbon isotope ratio of feathers reveals feeding behaviour of cormorants. The Auk 107, 400-437 (1990).
Peterson, B. J. & Fry, B. Stable isotopes in ecosystem studies. Ecology, Evolution, and Systematics 18, 293-320 (1987).
Rubenstein, D. R. & Hobson, K. A. From birds to butterflies: Animal movement patterns and stable isotopes. Trends in Ecology & Evolution 19, 256-263 (2004).
Schell, D. M. et al. Bowhead whale (Balaena mysticetus) growth and feeding as estimated by C techniques. Marine Biology 103, 433-443 (1989).
Smith, R. J. et al. Distinguishing between populations of fresh and salt-water harbor seals (Phoca vitulina) using stable-isotope ratios and fatty acids. Canadian Journal of Fisheries and Aquatic Sciences 53, 272-279 (1996).
Weber, P. K. et al. Otolith sulfur isotope method to reconstruct salmon (Oncorhynchus tshawytscha) life history. Canadian Journal of Fisheries and Aquatic Sciences 59, 587-591 (2002).
Werner, R. A. & Brand, W. A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Communications in Mass Spectrometry 15, 501-519 (2001).
West, J. B. et al. Stable isotopes as one of nature's ecological recorders. Trends in Ecology & Evolution 21, 408-414 (2006).






























