« Prev Next »
Biofilms are groups of attached bacteria, which are both resilient and adaptive. They often show remarkable organization and can communicate, coordinate, and cooperate with each other. The biofilm begins to dispel the notion that bacteria are simply many single cells out for themselves in favor of the idea that they can act as groups of cooperating cells to enhance their individual fitness and increase the efficiency of the biofilm. This is not altruism per se, but rather by exploiting the biofilm, individual fitness is enhanced by the interactions. As with any mutualism or commensalism, this does not rule out selection on individual cells. This notion has transformed microbiology, and forces us to rethink the roles of microbes and how they operate within the earth's many ecosystems.
Learning outcomes:
Upon reading this article, students will be able to:
1. Demonstrate a basic knowledge of biofilms by identifying their components, structure, and formation processes
2. Recognize biofilms as an adaptation strategy for bacteria to overcome environmental stresses
3. Articulate the abundance of bacteria and biofilms by describing their presence in natural and engineered environments.
Introduction.
Dominance of bacteria. Bacteria are too small to be seen by the naked eye and yet are the most diverse group of organisms on Earth. The individual cell averages just one micrometer in size (for context, an average human hair diameter is 100 micrometers) but owing to their sheer abundances, bacteria have the greatest biomass, outweighing all other forms of life put together (e.g., trees, whales, people). Bacteria also live within humans, and are essential to human health. Interestingly, a person has 10x more bacterial cells than human cells, and 100x more bacterial genes than human genes (Backhed et al. 2005). The human gut has over 1,000 different known species of bacteria, most of which are beneficial to our health. When acting together, they constitute a powerful and dynamic force that affects not only humans but also climate, nutrient cycling, and all known ecosystems.
From a bacterial cell's point of view, environments can change rapidly and frequently. For example, a bacterium traveling through the human gut can experience pHs ranging from highly acidic (pH 2-3) to basic (pH 8-9) in just a few hours. In hypersaline ponds, bacteria in microbial mats can experience salinities of 25-350 g/L, and periods of no water (desiccation). The bacteria of deep-sea hydrothermal vents can experience toxic metals coupled with temperatures changing from >100 oC to near freezing (0 oC) within minutes. Finally, bacteria experience varying levels of nutrients such as nitrate, phosphate, and sulfates, and the presence or absence of oxygen and water. Wherever bacteria live, they are exposed to many different stresses that compromise their ability to grow and even survive. To overcome these stresses, they have developed adaptive strategies that help stabilize their local environment and ecological success.
Biofilm Basics
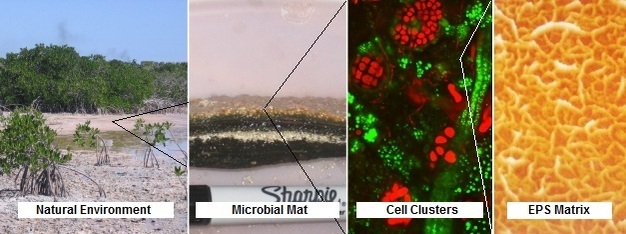
Why do bacteria exist mostly in a biofilm state? It is now realized that most bacteria live in a biofilm state, where cells aggregate together and surround themselves with a protective array of secreted molecules, called EPS (i.e., extracellular polymeric substances) (Figure 1). The biofilm is an adaptation for stabilizing the local environment of bacteria against stresses that is flexible and can be easily changed depending on conditions. For this reason, the EPS matrix surrounding bacteria has been referred to as the ‘house of biofilms' (Flemming and Wingender 2010). While the biofilm is not required by bacteria for survival, the biofilm state is an adaptation that enhances survival, metabolism, and propagation of bacteria, especially under adverse conditions.
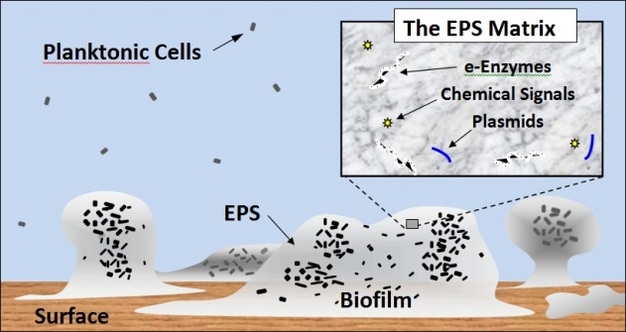
Definition, basics (components and formation process). A biofilm forms when one or several bacterial cells suspended in the water attach to a surface. At this point, the cell either detaches again or attaches more firmly by secretion of EPS. As the biofilm develops, different sets of genes are turned on (up-regulated) or off (down-regulated). Therefore bacteria in biofilms express genes differently when compared with similar bacteria as planktonic cells. Some of the genes that are up-regulated are involved in the secretion of EPS. The EPS matrix affords protection to cells and enhances gathering of nutrients through sorption of organics (Decho 1990). The EPS matrix forms the crucial three-dimensional architecture of a biofilm. This allows cells to fix their positions relative to one another, much in the same way that people live in different rooms of a high-rise apartment building. The EPS also forms a confining scaffold to slow diffusive loss and localize extracellular enzymes and extracellular DNA, which is actively secreted by cells as plasmids, or released by lysed cells. The proximity of cells to one another facilitates exchange of plasmids and free DNA, a process that can quickly arm bacteria with the necessary genetic machinery to persist through stresses. Finally, biofilm-forming bacteria use chemical communication, called quorum sensing, to coordinate their metabolism (Camilli and Bassler 2006). Bacteria release chemical signals that are sensed by other nearby cells within the biofilm. Thus, groups of bacteria in spatial proximity can coordinate their activities, a process that increases the efficiency and resilience of the community.
Overall, the biofilm state provides a more stable microenvironment for bacteria and other microbial cells. This microenvironment can rapidly adapt to stressors and coordinate activities in response to changes imposed by the environment, other organisms, or both. The species and metabolic diversity that typically develop within a biofilm afford the community greater adaptability and enhanced ecological persistence. These unique characteristics allow biofilms to be present in virtually every corner of natural or engineered environments and, often, to be an integral component of such environments. Some biofilms are found in extremely toxic environments and others act as the workhorse of man-made systems. Below are a few examples of the wide array of environments in which biofilms thrive.
Biofilms Found in Nature
Biofilms in hydrothermal vents
With their steep gradients of pH, temperature, pressure, and metals, deep-sea hydrothermal vents are an excellent example of the extreme natural conditions to whic microorganisms can physiologically adapt.. It appears that the formation of biofilms is a crucial strategy that microbes use to withstand this harsh environment (Edwards et al. 2005).
Hydrothermal vents occur globally at sea-floor spreading zones, where extremely hot and acidic (pH ~2-3) lavas and anoxic hydrothermal fluids, enriched in dissolved heavy metals and reactive gases mix with cold, oxygenated seawater to precipitate metal sulfides and form spectacular features such as black smokers (i.e., vents). These vents host a wide array of microbial communities and unique animals. Microbial biomass abundantly associates with vent chimneys of either sulfide or carbonate, and varies with the degree of hydrothermal activity (Schrenk et al. 2004; Schrenk et al. 2003; Sylvan et al. 2012) (Figure 2). Schrenk and colleagues (2003) reported that biofilms with a density of >108 cells/g were present within the porous walls of active deep-sea sulfide chimneys where temperatures exceed 100 °C (Schrenk et al. 2003).
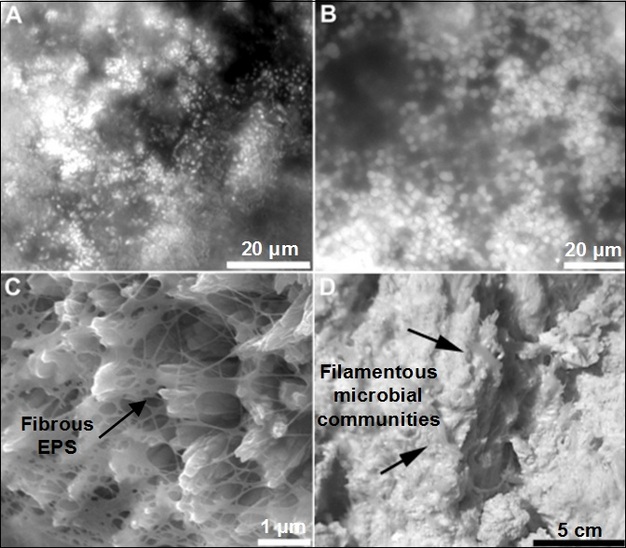
Biofilms and the plethora of organisms that live at deep-sea vent sites are based on the process of chemoautotrophy. Chemoautotrophs obtain energy by oxidizing organic molecules or inorganics (e.g., metals) to obtain energy, in contrast to phototrophs, which capture photons from sunlight to obtain energy for photosynthesis. Indeed, researchers believe that studying hydrothermal vents may aid in answering one of biology's most fundamental questions: What is the origin of life? (Martin et al. 2008; Edwards et al. 2011).
Other types of high-temperature microbial biofilm communities occur near the earth's surface at terrestrial hot springs such as those of Yellowstone National Park (Wyoming, USA). These thick biofilms, called microbial mats, are fueled by photosynthetic fixation of carbon into organic molecules, in contrast to the chemoautotrophic-based carbon fixation found at the deep-sea vents.
Biofilms in Engineering Systems
Corrosion — Should biofilms take the blame?
Biofilms are also common in engineered systems and may constitute both a problem for and a solution to engineering endeavors. For example, metal corrosion caused by biofilms is an issue of great concern to many industries ranging from oil/gas production to the distribution of safe drinking water. Mitigation of corrosion is estimated to cost 3% of the world's gross domestic product (~$1.9 trillion per year) (Koch et al. 2001). Despite the presence of residual disinfectant in treated drinking water, biofilms often develop on the inner surfaces of distribution pipes (Figure 3), possibly because the nutrient-poor nature of treated drinking water flowing through pipes facilitates biofilm formation as a physiological adaptation response. In such cases, biofilms are traditionally known to be responsible for causing or accelerating corrosion, a process known as biocorrosion or microbiologically-induced corrosion (MIC) (Beech 2004; Little et al. 2008; Belkaid et al. 2011; Wang et al. 2012). MIC is typically caused by the presence of biofilm-derived enzymes and acids that facilitate metal dissolution.
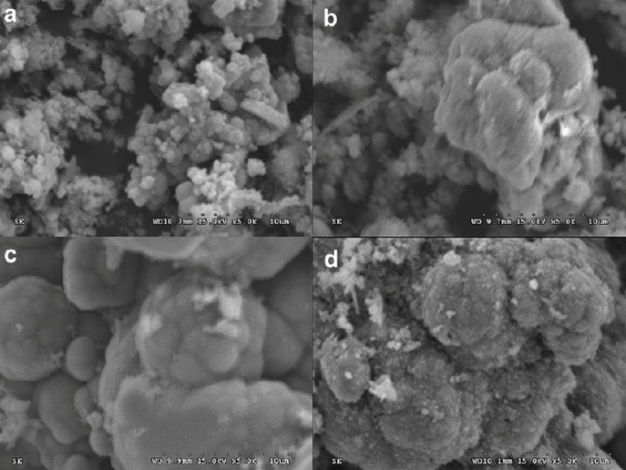
On the other hand, recent studies have shown that biofilms are also capable of suppressing corrosion (Zuo 2007; Stadler et al. 2008; Videla and Herrera 2009). This is because certain bacteria consume the resources (e.g., oxygen) needed for corrosion reactions to proceed. These contradictory reports suggest that relationships between corrosion and biofilms require better understanding of the diverse biofilm communities and of the factors that induce or inhibit corrosion. While some biofilm bacteria are troublemakers, others offer free and hardworking labor for humans.
Beneficial biofilms in water and wastewater treatment
Biofilms are widely used in environmental engineering systems as the powerhouse of treatment processes. For example, biofilms are used to remove organic matter in drinking water, a process called biologically active filtration (i.e., biofiltration). They are also used to remove biological nutrients in wastewater treatment (Figure 4). In both cases, bacteria are utilized for their ability to rapidly break down and transform complex organic compounds into simple forms that have fewer negative impacts on the receiving environment. Specifically, biofilm bacteria are much more efficient than planktonic cells in many treatment systems; the immobilized cells in biofilms will not be washed away, thus allowing for continuous treatment of large volumes of water. This engineering advantage also provides biofilm bacteria with a continuous source of food (i.e., carbon and other nutrients found in the untreated water), which allows the cells to thrive under an otherwise nutrient-poor condition.
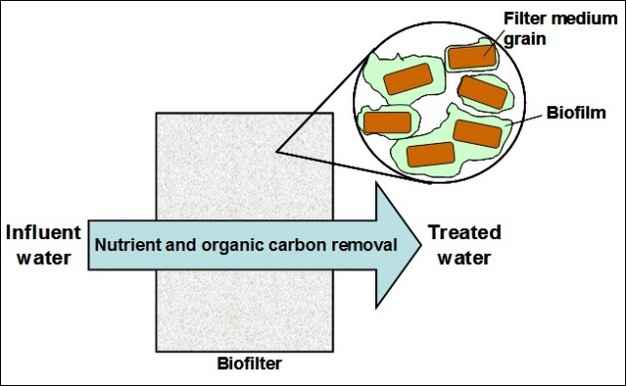
Biofiltration in drinking water treatment has been gaining increasing interest in recent years as a potential way to reduce the formation of disinfection byproducts (DBPs) (Hozalski et al. 1995; Zhu et al. 2010). DBPs are formed through chemical reactions between disinfectants, such as chlorine, and natural organic matter (NOM) (Hua and Reckhow 2007). While DBP formation can be reduced by changing the disinfectant used, another method is to decrease quantities of NOM in water using biofiltration, which occurs prior to disinfectant addition. Granular media filters can be converted to biofilters simply by allowing biofilm growth on media surfaces. These biofilms then break down NOM during filtration, resulting in much lower organic carbon levels that react with disinfectants to form DBPs. Another advantage of using biofiltration is that biofilm activity can also minimize taste and odor issues in drinking water by removing related organic compounds (Huck et al. 2000).
In wastewater treatment, nutrient removal is an essential outcome to protect the natural environment from eutrophication and unwanted contamination. Nutrient removal is achieved primarily by biological means; the most common is through the use of activated sludge, which is a mixture of flocs. Flocs are equivalent to biofilms formed in suspension, rather than attached to surfaces, where cells of various species are glued together by EPS, forming a suspended biofilm having a complex microbial community. This community of cells then breaks down a wide variety of organic compounds as well as nitrogen and phosphorous compounds. Similarly, surface-attached biofilms are used in some wastewater treatment plants in the form of trickling filters. These trickling filters are similar to biofilters in drinking water with the same objective of nutrient removal as the activated sludge process.
In all cases, the advantage of utilizing biofilms in water and wastewater treatments is attributed to the ‘stickiness' of the biofilm matrix. The complex and heterogeneous EPS matrix often captures and immobilizes organic and inorganic contaminants that need to be removed, such as pathogens, heavy metals, and nanoparticles (Searcy et al. 2006; Unz and Shuttleworth 1996; Peulen and Wilkinson 2011). The rapid physiological adaptation capabilities of biofilms also adds a major advantage: bacteria recover faster from stress caused by random occurrences of toxic substances in the influent water, thus enabling reliable water and wastewater treatment.
Summary
As a product of physiological adjustments made by microorganisms in response to environmental stressors, biofilms are ubiquitous in ecosystems, even under extreme conditions. Understanding biofilms is critical to helping solve questions ranging from ‘What is the origin of life?' to ‘How can water be cleaned?'
Acknowledgements
The support of the US National Science Foundation (Grant numbers BME-1032579 and DMR-1206072) is gratefully acknowledged.References and Recommended Reading
Backhed, F. et.al. Host-bacterial mutualism in the human intestine. Science 307, 1915-1920 (2005).
Flemming, H. C. & Wingender, J. The biofilm matrix. Nature Reviews Microbiology 8, 623-633 (2010).
Decho, A. W. Microbial exopolymer secretions in ocean environments--their role(s) in food webs and marine processes. Oceanography and Marine Biology Annual Review 28, 73-153 (1990).
Camilli, A. & Bassler, B. L. Bacterial small-molecule signaling pathways. Science 311, 1113-1116 (2006).
Edwards, K. J et.al.. Geomicrobiology in oceanography: microbe-mineral interactions at and below the seafloor. Trends in Microbiology 13, 449-456 (2005).
Schrenk, M. O et.al. A. Low archaeal diversity linked to subseafloor geochemical processes at the Lost City Hydrothermal Field, Mid-Atlantic Ridge. Environmental Microbiology 6, 1086-1095 (2004).
Schrenk, M. O et.al.. Incidence and diversity of microorganisms within the walls of an active deep-sea sulfide chimney. Applied and Environmental Microbiology 69, 3580-3592 (2003).
Sylvan, J. B. et. al. Life and Death of Deep-Sea Vents: Bacterial Diversity and Ecosystem Succession on Inactive Hydrothermal Sulfides. Mbio 3, 1 e00279 (2012).
Martin, W et.al.. Hydrothermal vents and the origin of life. Nature Reviews Microbiology 6, 805-814 (2008).
Edwards, K. J et.al.. Under the sea: microbial life in volcanic oceanic crust. Nature Reviews Microbiology 9, 703-712 (2011).
Koch, G. H et.al.. Corrosion Cost and Preventive Strategies in the United States, Federal Highway Administration, Office of Infrastructure Research and Development (2001).
Beech, I. B. Corrosion of technical materials in the presence of biofilms--current understanding and state-of-the art methods of study. International Biodeterioration and Biodegradation 53, 177-183 (2004).
Little, B. J et.al.. The influence of marine biofilms on corrosion: A concise review. Electrochimica Acta 54, 2-7 (2008).
Belkaid, S et.al. Effect of biofilm on naval steel corrosion in natural seawater. Journal of Solid State Electrochemistry 15, 525-537 (2011).
Wang, H. B et.al. Effects of disinfectant and biofilm on the corrosion of cast iron pipes in a reclaimed water distribution system. Water Research 46, 1070-1078 (2012).
Zuo, R. Biofilms: strategies for metal corrosion inhibition employing microorganisms. Applied Microbiology and Biotechnology 76, 1245-1253 (2007).
Stadler, R., et al. First evaluation of the applicability of microbial extracellular polymeric substances for corrosion protection of metal substrates. Electrochimica Acta 54, 91-99 (2008).
Videla, H. A. & Herrera, L. K. Understanding microbial inhibition of corrosion. A comprehensive overview. International Biodeterioration and Biodegradation 63, 896-900 (2009).
Hozalski, R. M et.al.TOC removal in biological filters. Journal of American Water Works Association 87, 40-54 (1995).
Zhu, I. X et.al.. Review of biologically active filters in drinking water applications. Journal of American Water Works Association 102, 67-77 (2010).
Hua, G. & Reckhow, D. A. Characterization of disinfection byproduct precursors based on hydrophobicity and molecular size. Environmental Science and Technology 41, 3309-3315 (2007).
Huck, P. M et.al. Optimizing filtration in biological filters, AWWA Research Foundation and American Water Works Association (2000).
Searcy, K. E et. al. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology 72, 6242-6247 (2006).
Unz, R. F. & Shuttleworth, K. L. Microbial mobilization and immobilization of heavy metals. Current Opinions in Biotechnology 7, 307-310 (1996).
Peulen, T. O. & Wilkinson, K. J. Diffusion of nanoparticles in a biofilm. Environmental Science and Technology 45, 3367-3373 (2011).






























