« Prev Next »

Until recently, the evolutionary events that surrounded the origin of the hominin lineage — which includes modern humans and our fossil relatives — were virtually unknown, and our phylogenetic relationship with living African apes was highly debated. Gorillas and chimpanzees were commonly regarded to be more closely related to each other due to their high degree of morphological and behavioral similarities, such as their shared mode of locomotion — knuckle-walking. But with the advent of molecular studies it has become clear that chimpanzees share a more recent common ancestor with humans, and are thus more closely related to us than they are to gorillas (e.g., Bailey 1993, Wildman et al. 2003). The similarities between the living African apes were thought to have been inherited from a common ancestor (=primitive features), implying that the earliest hominins and our last common ancestor shared with chimpanzees had features that were similar, morphologically and behaviorally, to the living African apes (Lovejoy 2009). With the discoveries of the earliest hominin species discussed below, it is now possible to critically examine these assumptions.
The chimpanzee-human divergence date has been estimated to be between 8 and 5 million years ago (MA) since the 1960s through immunologic and molecular techniques (e.g., Steiper & Young, 2006). Driven largely in part by these new genetic-based hypotheses, there have been intensive efforts by different teams over the last two decades to find and explore sediments that record this crucial time period for which we had virtually no fossil evidence. Their hard work and perseverance led to the discovery of several new genera and species of early hominins that are dated close to the estimated divergence dates for chimpanzees and humans. In 1994, Ardipithecus ramidus (ca. 4.4 Ma) was announced (White et al. 1994, 1995, WoldeGabriel et al. 1994) and soon after, even older hominins were discovered: Orrorin tugenensis (6.0-5.7 Ma, Pickford & Senut 2001, Senut et al. 2001, Sawada et al. 2002), Sahelanthropus tchadensis (7-6 Ma, Brunet et al. 2002, 2005, Vignaud et al. 2002), and Ardipithecus kadabba (5.8-5.2 Ma, Haile-Selassie 2001, WoldeGabriel et al. 2001).
These earliest hominins lack derived features found in later hominins, and their inclusion in the hominin lineage is largely based on a reduction in canine size, absence of the C/P3 honing complex, and the presence of morphological adaptations for habitual or obligate bipedality generally found in the postcranial skeleton, particularly in the pelvis and hindlimb. Bipedality is often considered to be the hallmark of hominins, and its presence in fossil species is often the key to their inclusion in the hominin clade. However, there is much contention and their status as hominins is contested by various researchers in the field (Wolpoff et al. 2002, 2006, Andrews & Harrison, 2005, Harrison, 2010, Wood & Harrison, 2011).
Sahelanthropus tchadensis
Sahelanthropus tchadensis from the site of Toros-Menalla, Chad (Figure 1), discovered by the Mission Paléoanthropologique Franco-Tchadienne (Brunet et al. 2002), may be the oldest hominin recovered thus far. However, the age of Sahelanthropus was first determined biochronologically (Vignaud et al. 2002). Unlike eastern African sites, Toros-Menalla lacks volcanic tuffs, which precludes the use of radiometric dating. Although an age range of 7–6 Ma is suggested for Sahelanthropus, cosmogenic nuclide dating using 10Be indicates that the sediments from which the fossils derive are at the older end of that range at 7.2–6.8 Ma (Lebatard et al. 2008).

Specimens of Sahelanthropus recovered include a complete, but crushed, cranium (TM 266-01-60-1), isolated mandibular fragments and dentition (Brunet et al. 2002, 2005). While the cranium possesses a host of primitive characters, it is argued to share derived features with later hominins that confirm its status as a member of the hominin clade. These include small canines worn at the tip, implicative of a reduced or absent C/P3 honing complex, and a short cranial base with a foramen magnum that is positioned anteriorly and orthogonal to the orbital plane, suggestive of an upright posture and habitual bipedality (Figure 2, Brunet et al. 2002, Guy et al. 2005, Zollikofer et al. 2005).

There is, however, much contention that Sahelanthropus is a hominin based on these traits. The determination of the small relative size of the canine is dependent on the supposition that the cranium is male as inferred from its large browridges. Various researchers have suggested that the link between browridge size and sex is tenuous — thus, a more parsimonious conclusion is that the cranium belongs to a female individual (Wolpoff et al. 2002, Andrews & Harrison 2005), specifically that of a female ape (and not a hominin at all) in whom canines are more likely to be worn at the tip (Wolpoff et al. 2002, 2006). They further argue that without diagnostic postcranial elements with clear adaptations for obligate bipedalism, such as the pelvis and femur, any inference made about the positional and locomotor behavior of Sahelanthropus is premature, because the taxonomic value and functional significance of shortened cranial base and foramen magnum position are unclear (Wolpoff et al. 2002, 2006, Andrews & Harrison 2005, Wood & Harrison 2011).
Orrorin tugenensis
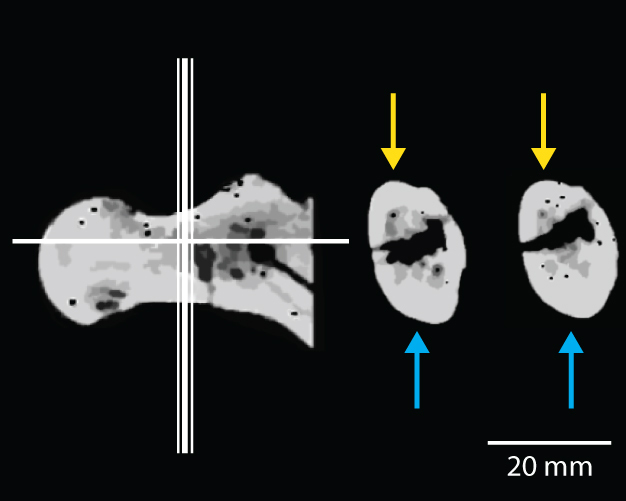
As one of the postcranial elements that shows diagnostic features of habitual bipedality, BAR 1002'00, a proximal femur, has been at the center of the debate. It shares several traits with later hominins that are interpreted as indicators of bipedality: elongated and antero-posteriorly compressed femoral neck, thicker cortex inferiorly than superiorly in the femoral neck, presence of an obturator externus groove, and well-developed gluteal tuberosity (Senut et al. 2001, Pickford et al. 2002, Galik et al. 2004). There has been particular emphasis on the asymmetric cortical bone distribution in the femoral neck, which is considered one of the key traits indicative of bipedality in Orrorin (Figure 3, Pickford et al. 2002, Galik et al. 2004). However, many researchers questioned the methodology employed and the quality of the CT scans used to demonstrate the inferiorly thicker femoral neck cortex of Orrorin (Ohman et al. 2005, White 2006), while others criticized the usefulness of the trait in general, regardless of its presence in Orrorin, as an indicator of habitual or obligate bipedality (Andrews & Harrison 2005). Senut et al. (2001) and Pickford et al. (2002) also claimed that the Orrorin femora were more human-like than Australopithecus species, and argued, based on this, that Orrorin led directly to Homo — thus, relegating Australopithecus to a side branch of the human evolutionary tree. However, recent morphometric study by Richmond & Jungers (2008) on BAR 1002'00 suggests that Orrorin is morphologically similar to Pliocene hominins, distinct from Homo and modern great apes, implying that it may have possessed a form of bipedality similar to that of Pliocene hominins.
Ardipithecus kaddaba
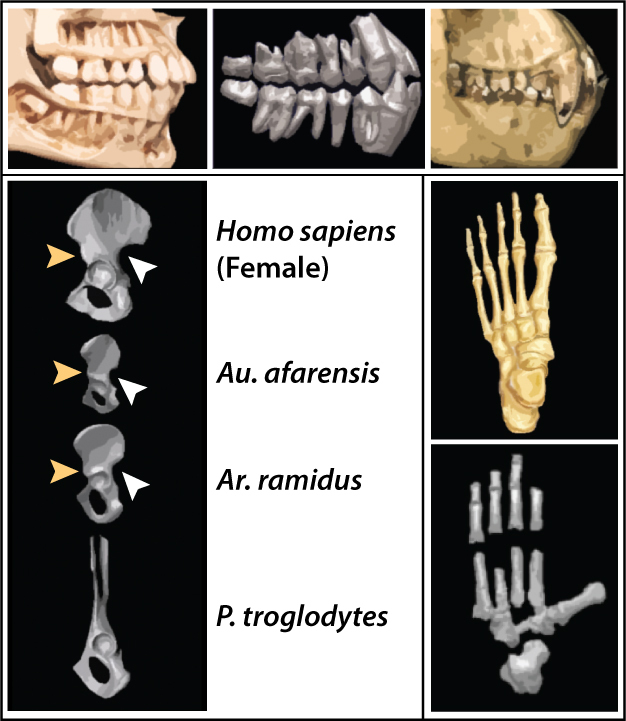
Ardipithecus kaddaba is known from the late Miocene localities of the western margin of Middle Awash, Ethiopia (Figure 1). Specimens consist of mandibular fragments, isolated teeth and few postcranial elements recovered from the Asa Koma (5.54–5.77 Ma) and Kuseralee (ca. 5.2 Ma) Members of the Middle Awash study area (Haile-Selassie 2001, WoldeGabriel et al. 2001, Haile-Selassie et al. 2004, Renne et al. 2009). They were originally referred to as a subspecies of Ardipithecus ramidus (Haile-Selassie 2001), but were later elevated to a species based primarily on the primitive morphology of the C/P3 complex that implied the potential for some functional honing (Figure 4, Haile-Selassie et al. 2004, 2009). Among the postcranial elements recovered is a pedal proximal phalanx on which the proximal articular surface is dorsally canted (Figure 4). This feature is associated with toeing-off and is unique to bipeds (Latimer & Lovejoy 1990) — thus, linking Ar. kadabba to later hominins (Haile-Selassie 2001, Haile-Selassie et al. 2009). However, the fact that the pedal phalanx was recovered from the younger Kuseralee Member, while the dentition was from the older Asa Koma Member have prompted some doubts regarding its association to Ar. kadabba (Begun 2004).

Ardipithecus ramidus
The main traits that link Ar. ramidus to later hominins include: small, blunt canines, reduced canine size dimorphism, lack of a functional C/P3 honing complex, anteriorly positioned foramen magnum, and characters inferred to be indicative of bipedality, such as the presence of a greater sciatic notch, anterior inferior iliac spine, inferred lumbar lordosis, and dorsal canting of the pedal phalanx (Figure 5, White et al. 1994, 2009a, Semaw et al. 2005; Suwa et al. 2009a, 2009b, Lovejoy et al. 2009b, 2009c). However, "Ardi" also shows a number of primitive characters, particularly in the postcranium: fully opposable big toe, absence of longitudinal arch in the foot, relatively equal fore- to hind-limb lengths, and ape-like lower pelvis (Figure 5, White et al 2009a, Lovejoy et al. 2009a, 2009b, 2009c, 2009d). Thus, "Ardi" shows an unexpected mosaic of derived and primitive features that suggest it was a facultative biped, able to climb in the trees effectively by palmigrade quadrupedalism (Lovejoy et al. 2009d). This refutes the previous assumptions that the last common ancestor was chimpanzee-like, and instead suggests that living chimpanzees are highly specialized (Lovejoy et al. 2009d).
It has been noted, however, that Oreopithecus bambolii, a late Miocene ape from Italy, shows many of the characters that are used to denote the hominin status of Ar. ramidus. These include relatively small canines, reduction of C/P3 honing complex, anteriorly placed foramen magnum, and well-developed anterior inferior iliac spine. While other morphological features of Oreopithecus leave no doubt that it is an ape, the presence of the above characters suggests that interpretations of the earliest hominins may be confounded by homoplasies (Wood & Harrison 2011).
Paleoenvironment of the Earliest Hominins
Current interpretations of the paleoenvironment suggest that Orrorin is associated with open woodland habitats with dense woodland or forest in the vicinity, possibly along lake margins (Pickford & Senut 2001). Sahelanthropus is likely found in a mosaic of environments, ranging from gallery forest at the edge of a lake, to savanna woodland, to open grassland, although there are indications that there was a dominance of shrub/bushland and grassy woodland habitats within the Chadian lake basin (Vignaud et al. 2002, Le Fur et al. 2009, Blondel et al. 2010). Ardipithecus kadabba is associated with riparian woodland and floodplain grassland along water margins (Su et al. 2009). Ardipithecus ramidus is found in closed woodland habitats with possible patches of forest at Aramis (White et al. 2009b, but see Cerling et al. 2010 for a different view) and associated with bushland and grassland habitats at Gona (Levin et al. 2008). Combined, these paleoenvironmental interpretations of the African latest Miocene and earliest Pliocene suggest that the beginnings of our lineage did not occur in open, semi-arid to arid habitat conditions, but rather in more closed and/or wet habitats. However, a definitive conclusion is difficult to draw at this time given the lack of detailed paleoecological reconstruction for Orrorin, the possibility that Sahelanthropus was found in more open habitats, the discordance in interpretation of the Aramis dataset, and the general paucity of late Miocene hominin-bearing sites in Africa.
While the hominin status of Sahelanthropus, Orrorin, and Ardipithecus species may be debated, their significance for understanding human origins and evolution is clear. They open a window into the very beginnings of our lineage, at the time when our ancestors diverged from those of our chimpanzee cousins. As more fossils of these species and their associated fauna are recovered and analyzed, we will learn more not only about their paleobiology, taxonomy, and phylogenetic relationships, but also about their environment, and how that may have influenced the evolution of our lineage.
Glossary
C/P3 honing complex: Refers to the arrangement of the upper canine and lower third premolar that allows the back edge of the upper canine to be sharpened or honed against the front edge of the lower premolar.
Foramen magnum: A large hole at the underside of the skull where the spinal cord enters the skull to attach to the brain.
Gluteal tuberosity: Located on the back surface of the femur where part of the gluteus maximus muscle inserts. Its expression is variable, ranging from a depression to a tuberosity.
Homoplasy: A trait shared by different species due to shared function rather than shared ancestry, i.e. the trait is not present in their last common ancestor.
Lumbar lordosis: Inward curvature of the spinal column at the lumbar vertebrae (= lower back). Among primates, this is seen only in bipedal hominins.
Miocene: Period in the geologic time scale that extends from 23 to 5.3 million years ago.
Obturator externus groove: A shallow groove on the back surface of the neck of the femur. In hominins, habitual bipedal posture brings the insertion tendon of the obturator externus muscle into contact with the back surface of the femoral neck, creating the groove.
Palmigrade quadrupedalism: Mode of locomotion where an organism moves on all four limbs and support in the forelimb is on the palmar surface.
Phylogenetic relationship: Relationship between groups of organisms based on evolutionary history, i.e shared common ancestors.
Pliocene: Period in the geologic time scale that extends from 5.3 to 2.6 million years ago. Prior to a revision of the time scale in 2009, the Pliocene extended from 5.3 to 1.8 million years ago.
Radiometric dating: Absolute dating techniques that use the ratio of naturally occurring radioactive isotope and its decay product.
References and Recommended Reading
Andrews, P. & Harrison, T. "The last common ancestor of apes and humans," in Interpreting the Past: Essays on Human, Primate, and Mammal Evolution, eds. D. E. Lieberman et al. (Boston, MA, and Lieden, Netherlands: Brill Academic Publishing, 2005) 103-121.
Bailey, W. J. Hominoid trichotomy: A molecular overview. Evolutionary Anthropology: Issues, News, and Reviews 2, 100-108 (1993).
Blondel, C. et al. Dental mesowear analysis of the late Miocene Bovidae from Toros-Menalla (Chad) and early hominin habitats in Central Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 292, 184-191 (2010).
Brunet, M. et al. A new hominin from the upper Miocene of Chad, Central Africa. Nature 418, 145-151 (2002).
Brunet, M. et al. New material of the earliest hominin from the upper Miocene of Chad. Nature 434, 752-755 (2005).
Cerling, T. E. et al. Comment on the paleoenvironment of Ardipithecus ramidus. Science 328, 1105 (2010).
Dart, R. A. The osteodontokeratic culture of Australopithecus prometheus. Transvaal Museum (Pretoria) Memoir 10, 1-105 (1957).
Galik, K. et al. External and internal morphology of the BAR 1002'00 Orrorin tugenensis femur. Science 305, 1450-1453 (2004).
Guy, F. et al. Morphological affinities of the Sahelanthropus tchadensis (late Miocene hominin from Chad) cranium. Proceedings of the National Academy of Sciences of the United States of America 102, 18836-18841 (2005).
Haile-Selassie, Y. et al. "Homininae," in Ardipithecus kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia, eds. Y. Haile-Selassie & G. WoldeGabriel (Berkeley, CA, and London, UK: University of California Press, 2009) 159-236.
Haile-Selassie, Y. et al. Late Miocene teeth from Middle Awash, Ethiopia, and early hominin dental evolution. Science 303, 1503-1505 (2004).
Haile-Selassie, Y. Late Miocene hominins from the Middle Awash, Ethiopia. Nature 412, 178-181 (2001).
Harrison, T. Apes among the tangled branches of human origins. Science 327, 532-534 (2010).
Latimer, B. & Lovejoy, C.O. Hallucal tarsometatarsal joint in Australopithecus afarensis. American Journal of Physical Anthropology 82, 125-133 (1990).
Lebatard, A-E. et al. Cosmogenic nuclide dating of Sahelanthropus tchadensis and Australopithecus bahrelghazali: Mio-Pliocene hominins from Chad. Proceedings of the National Academy of Sciences of the United States of America 105, 3226-3231 (2008).
Le Fur, S. et al. The mammal assemblage of the hominid site TM266 (Late Miocene, Chad Basin): Ecological structure and paleoenvironmental implications. Naturwissenschaften 96, 565-574 (2009).
Levin, N. E. et al. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. The Geological Society of America Special Paper 446, 215-234 (2008).
Lovejoy, C. O. Reexamining human origins in light of Ardipithecus ramidus. Science 326, 74e1-74e8 (2009).
Lovejoy, C. O. et al. Careful climbing in the Miocene: The forelimbs of Ardipithecus ramidus and humans are primitive. Science 326, 70e1-70e8 (2009a).
Lovejoy, C. O. et al. The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science 326, 71e1-71e6 (2009b).
Lovejoy, C. O. et al. Combining prehension and propulsion: The foot of Ardipithecus ramidus. Science 326, 72e1-72e8 (2009c).
Lovejoy, C. O. et al. The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science 326, 100-106 (2009d).
Ohman, J. C. et al. Questions about Orrorin femur. Science 307, 845 (2005).
Pickford, M. & Senut, B. The geological and faunal context of late Miocene hominin remains from Lukeino, Kenya. Comptes Rendus Académie de la Terres et des Planètes 332, 145-152 (2001).
Pickford, M. et al. Bipedalism in Orrorin tugenensis revealed by its femora. Comptes Rendus Palevol 1, 191-203 (2002).
Renne, P. R. et al. "Geochronology," in Ardipithecus kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia, eds. Y. Haile-Selassie & G. WoldeGabriel (Berkeley, CA, and London, UK: University of California Press, 2009) 93-103.
Richmond, B. G. & Jungers, W. L. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science 319, 1662-1665 (2008).
Sawada, Y. et al. The age of Orrorin tugenensis, an early hominin from the Tugen Hills, Kenya. Comptes Rendus Palevol 1, 293-303 (2002).
Semaw, S. et al. Early Pliocene hominins from Gona, Ethiopia. Nature 433, 301-305 (2005).
Senut, B. et al. First hominin from the Miocene (Lukeino Foramtion, Kenya). Comptes Rendus Académie de la Terres et des Planètes 332, 137-144 (2001).
Steiper, M. E. & Young, N. M. Primate molecular divergence dates. Molecular Phylogenetics and Evolution 41, 384-394 (2006).
Su, D. F. et al. "Paleoenvironment," in Ardipithecus kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia, eds. Y. Haile-Selassie & G. WoldeGabriel (Berkeley, CA, and London, UK: University of California Press, 2009) 521-547.
Suwa, G. et al. The Ardipithecus skull and its implications for hominin origins. Science 326, 68e1-68e7 (2009a).
Suwa, G. et al. Paleobiological implications of the Ardipithecus ramidus dentition. Science 326, 94-99 (2009b).
Vignaud, P. et al. Geology and palaeontology of the upper Miocene Toros-Menalla hominin locality, Chad. Nature 418, 152-155 (2002).
White, T. D. et al. Australopithecus ramidus, a new species of early hominin from Aramis, Ethiopia. Nature 371, 306-312 (1994).
White, T. D. et al. Corrigendum: Australopithecus ramidus, a new species of early hominin from Aramis, Ethiopia. Nature 375, 88 (1995).
White, T.
D. Early hominin femora: The inside story. Comptes Rendus Palevol 5,
99-108 (2006).
White, T. D. et al. Ardipithecus ramidus and the paleobiology of early hominins. Science 326, 75-86 (2009a).
White, T. D. et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326, 87-93 (2009b).
Wildman, D. E. et al. Implications of natural selection in shaping 99.4% nonsynonymous DNA identity between humans and chimpanzees: Enlarging genus Homo. Proceedings of the National Academy of Sciences of the United States of America 100, 7181-7188 (2003).
WoldeGabriel, G. et al. Ecological and temporal placement of early Pliocene hominins at Aramis, Ethiopia. Nature 371, 330-333 (1994).
WoldeGabriel, G. et al. The geological, isotopic, botanical, invertebrate, and lower vertebrate surroundings of Ardipithecus ramidus. Science 326, 65e1-65e5 (2009).
WoldeGabriel, G. et al. Geology and paleontology of the late Miocene Middle Awash valley, Afar rift, Ethiopia. Nature 412, 175-178 (2001).
Wolpoff, M. H. et al. Sahelanthropus or ‘Sahelpithecus'? Nature 419, 581-582 (2002a).
Wolpoff, M. H. et al. An ape or the ape: Is the Toumaï cranium TM 266 a hominin? PaleoAnthropology 2006, 36-50 (2006b). doi:
Wood, B. & Harrison, T. The evolutionary context of the first hominins. Nature 470, 347-352 (2011).
Zollikofer, C. P. E. et al. Virtual cranial reconstruction of Sahelanthropus tchadensis. Nature 434, 755-759 (2005).






























