« Prev Next »
The Law of Conservation of Mass
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction. If we account for all reactants and products in a chemical reaction, the total mass will be the same at any point in time in any closed system. Lavoisier's finding laid the foundation for modern chemistry and revolutionized science.
The Law of Conservation of Mass holds true because naturally occurring elements are very stable at the conditions found on the surface of the Earth. Most elements come from fusion reactions found only in stars or supernovae. Therefore, in the everyday world of Earth, from the peak of the highest mountain to the depths of the deepest ocean, atoms are not converted to other elements during chemical reactions. Because of this, individual atoms that make up living and nonliving matter are very old and each atom has a history. An individual atom of a biologically important element, such as carbon, may have spent 65 million years buried as coal before being burned in a power plant, followed by two decades in Earth's atmosphere before being dissolved in the ocean, and then taken up by an algal cell that was consumed by a copepod before being respired and again entering Earth's atmosphere (Figure 1). The atom itself is neither created nor destroyed but cycles among chemical compounds. Ecologists can apply the law of conservation of mass to the analysis of elemental cycles by conducting a mass balance. These analyses are as important to the progress of ecology as Lavoisier's findings were to chemistry.
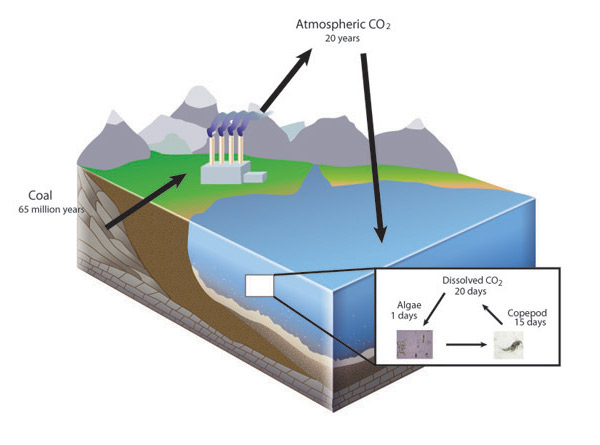
Life and the Law of Conservation of Mass

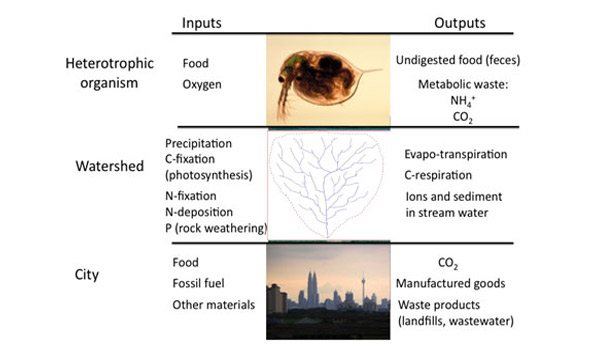
Ecosystems can be thought of as a battleground for these elements, in which species that are more efficient competitors can often exclude inferior competitors. Though most ecosystems contain so many individual reactions, it would be impossible to identify them all, each of these reactions must obey the Law of Conservation of Mass — the entire ecosystem must also follow this same constraint. Though no real ecosystem is a truly closed system, we use the same conservation law by accounting for all inputs and all outputs. Scientists conceptualize ecosystems as a set of compartments (Figure 2) that are connected by flows of material and energy. Any compartment could represent a biotic or abiotic component: a fish, a school of fish, a forest, or a pool of carbon. Because of mass balance, over time the amount of any element in any one of these compartments could hold steady (if inputs = outputs), increase (if inputs > outputs), or decrease (if inputs < outputs). For example, early successional forests gain biomass as trees grow and thus act as a carbon sink (Figure 3). In mature forests, the amount of carbon taken up through photosynthesis may equal the amount of carbon respired by the forest ecosystem, so there is no net change in stored carbon over time. When a forest is cut (and especially if trees are burned to clear land for agriculture), this stored carbon reenters the atmosphere as CO2. Mass balance ensures that the carbon formerly locked up in biomass must go somewhere; it must reenter some other compartment of some ecosystem. Mass balance properties can be applied over many scales of organization, including the individual organism, the watershed, or even a whole city (Figure 4).
Mass Balance of Elements in Organisms

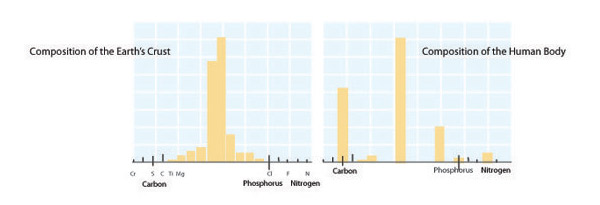
Each organism has a unique, relatively fixed, elemental formula, or composition determined by its form and function. For instance, large size or defensive structures create particular elemental demands. Other biological factors such as rapid growth can also influence elemental composition. Ribonucleic acid (RNA) is the biomolecular template used in protein synthesis. RNA has a high phosphorus content (~9% by mass), and in microbes and invertebrates RNA accounts for a large fraction of an organism's total phosphorus content. As a result, fast-growing organisms such as bacteria (which can double more than 6 times per day) have especially high phosphorus content and therefore demands. By contrast, among vertebrates structural materials such as bones (made of calcium phosphate) account for the majority of an organism's phosphorus content. Among mammals, black-tailed deer (Odocoileus columbianus; Figure 6) have a relatively high phosphorus demand due to their annual investment in calcium- and phosphorus-rich antlers. Failure to meet elemental demands can lead to poor health, limited reproduction, and even extinction. The extinction of the majestic Irish Elk (Megaloceros giganteus) is thought to have been caused by the shortened growing season that occurred during the last ice age, which reduced the availability of the calcium and phosphorus these animals needed to grow their enormous antlers.
Obtaining the resources required for metabolism, growth, and reproduction is one of the central challenges of life. Animals, particularly those that feed on plants (herbivores) or detritus (detritivores), often consume diets that do not include enough of the nutrients they need. The struggle to obtain nutrients from poor quality diets influences feeding behavior and digestive physiology and has led to epic migrations and seemingly bizarre behavior such as geophagy (feeding on materials such as clay and chalk). For example, the seasonal mass migration of Mormon crickets (Anabrus simplex) across western North America in search of two nutrients: protein and salt. Researchers have shown that the crickets stop walking once their demand for protein is met (Figure 7).
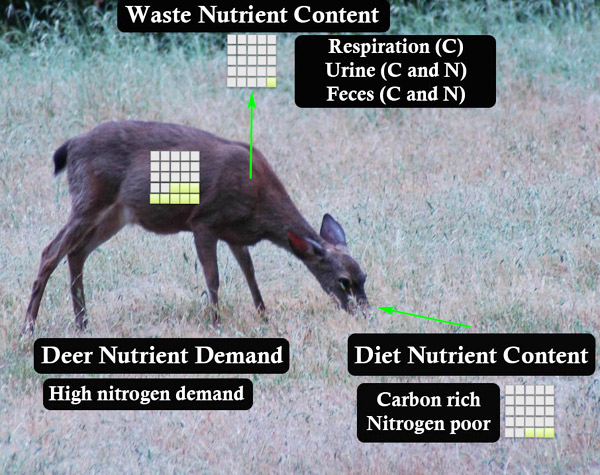
The flip side of the struggle to obtain scarce resources is the need to get rid of excess substances. Herbivores often consume a diet rich in carbon — think potato chips, few nutrients but lots of energy. Some of this material can be stored internally, but this is a limited option and excess carbon storage can be harmful, just as obesity is harmful to humans. Thus, animals have several mechanisms for getting rid of excess elements. Excess nutrients are released in feces or urine or sometimes it is respired (i.e., released as carbon dioxide). This release of excess nutrients can influence both food webs and nutrient cycles.
Mass Balance in Watersheds
Ecologists have often used naturally delineated ecosystems, such as lakes or watersheds, for applying mass balances. A forested watershed receives inputs of carbon through photosynthesis, inputs of nitrogen from nitrogen-fixing bacteria, as well as through the deposition of atmospheric nitrogen, inputs of phosphorus from the slow weathering of bedrock, and inputs of water from precipitation. Outputs include gaseous pathways (e.g., H2O losses through evapotranspiration, CO2 production as respiration, N2 produced by denitrifying bacteria) and dissolved pathways (nutrients and carbon dissolved in stream water). Outputs also include material transport across ecosystem boundaries, such as the movement of migratory animals or harvesting trees in a forest.

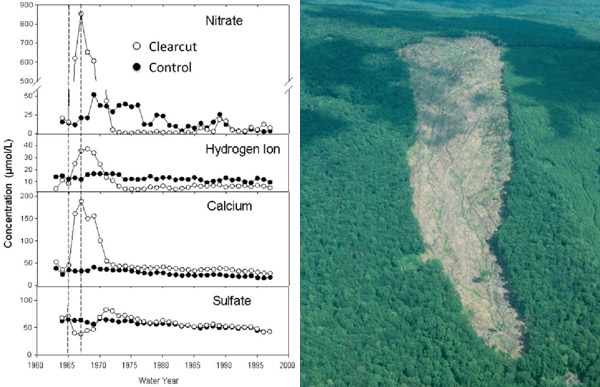
The Hubbard Brook Experimental Forest in the White Mountains of New Hampshire, USA, has been the site of ecosystem mass balance studies since the 1960s. This landscape has similar-sized, discreet watersheds drained by streams and underlain by impermeable bedrock. By installing V-notch weirs, investigators could precisely and continuously measure stream discharge. By measuring the concentration of nutrients and ions in stream water, they could quantify the losses of these materials from the ecosystem. After calculating inputs to the ecosystem (by sampling precipitation, dry deposition, and nitrogen fixation), they could also construct mass balances. Additionally, researchers could experimentally manipulate these watersheds to measure the effects of disturbance on nutrient retention. In 1965, an entire experimental watershed was whole-tree harvested, resulting in large increases in nitrate and calcium losses relative to an uncut reference watershed (Figure 8). By studying inputs and outputs, an understanding of the internal functioning of the ecosystem within the watershed was obtained.
Mass Balance in Human-Dominated Ecosystems
Mass balance constraints apply everywhere, even to highly altered ecosystems such as cities or agricultural fields. Cities import food, fuel, water, and other materials and export materials such as manufactured goods. Cities also produce large quantities of waste products — with solid waste sent to landfills, CO2 (and other pollutants) produced from the combustion of fossil fuels being released to the atmosphere. Nutrients from sewage and from fertilizer runoff can end up in rivers where they will fertilize downstream aquatic ecosystems.
Human agricultural systems can also be analyzed using a mass-balance, ecosystem approach. Traditional agricultural practices emphasized efficiency, with most production staying on the farm — food for livestock was produced on the farm, food for farmers' families was produced on the farm, and plant and animal waste was composted for use as fertilizer on the farm. As a result, the amount of material cycling within the farm "ecosystem" was large relative to the inputs and outputs to the system (a relatively closed ecosystem). By contrast, modern industrial agriculture emphasizes maximizing yields over efficiency. Farmers import fertilizer in large amounts (often far exceeding the amounts that crops can use) and grow and export commodity crops. Ironically, in these highly open ecosystems (where inputs and outputs can far exceed internal cycling), food for farmers' families must often be imported as well. Highly productive agricultural systems are critical in feeding the world's growing human population, but as many of the ingredients of modern agriculture (e.g., water, petroleum, phosphorus) become increasingly limiting over the next century (due to depleted geologic deposits), we will be faced with the challenge of increasing the efficiency of these systems. Just as the constraints of mass balance provide a useful tool for ecologists in studying natural ecosystems, mass balance also ensures that the increase in human population and material consumption that has characterized the past 200 years cannot continue indefinitely.
References and Recommended Reading
Chapin, F. S. et al. Principles of Terrestrial Ecosystem Ecology. New York, NY: Springer, 2002.
Likens, G. E. & Bormann, F. H. Biogeochemistry of a Forested Ecosystem. 2nd ed. New York, NY: Springer-Verlag, 1995.
Moen, R. A. et al. Antler growth and extinction of Irish Elk. Evolutionary Ecology Research 1, 235–249 (1999).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton, NJ: Princeton University Press, 2002.
Simpson, S. J. et al. Cannibal crickets on a forced march for protein and salt. Proceedings of the National Academy of Sciences of the USA 103, 4152-4156 (2006).































