« Prev Next »
Darwin's "Mystery of Mysteries"
"... these forms may still be only ... varieties; but we have only to suppose the steps of modification to be more numerous or greater in amount, to convert these forms into species ... thus species are multiplied" (Darwin 1859, p. 120).
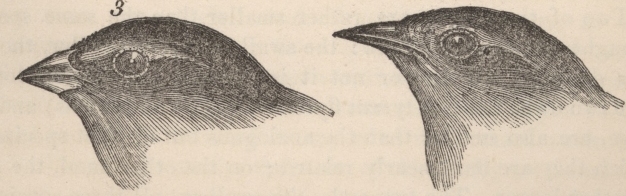
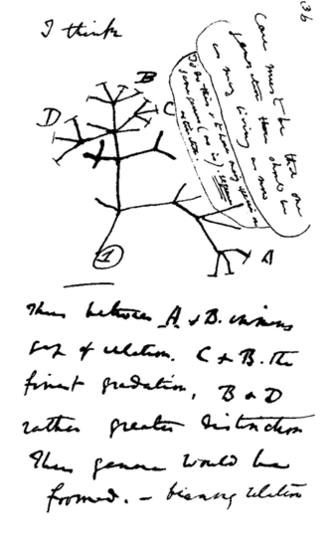
Discussion of most topics within Evolutionary Biology begins with Darwin. Indeed, On The Origin of Species (1859) continues to influence much of modern Evolutionary Biology. Darwin viewed evolution by natural selection as a very gradual mechanism of change within populations, and postulated that new species could be the product of this very same process, but over even longer periods of time. This eventual process of speciation by natural selection is illustrated by a sketch drawn by Darwin in his personal notebook nearly 20 years before the Origin of Species was published (Figure 1). Here, he proposed a model whereby lineages form from their ancestors by evolving different characters over relatively long periods of time. Darwin indicated that species could form by the evolution of one species splitting into two, or via a population diverging from its extant ancestor to the point it was a new species. Darwin's insights into evolution were brilliant, especially in light of their being made in the absence of genetics. Indeed, ideas about heredity and the introduction of new genetic material via mutation were to come long after Darwin's founding theories of evolution.
The Modern Synthesis
A major turning point for evolutionary research occurred in the 1930s when Fisher, Haldane, Wright, Dobzhansky, and others, developed mathematical population genetic models to illuminate the genetic mechanisms of evolutionary change (Mayr & Provine 1998). The integration of genetics with models of natural selection shed tremendous light on, and strengthened Darwin's views on, evolution — here was the missing mechanism that introduced new variation into populations via mutation and recombination. Indeed, thanks to the Modern Synthesis, much of current research in Evolutionary Biology is strongly tied to genetics, and current methods for studying speciation are no exception. As discussed below, the Modern Synthesis led to advances not only in the study of evolution within populations, but also changes in the way species were defined, and in how new species were considered to form.
Barriers to reproduction.
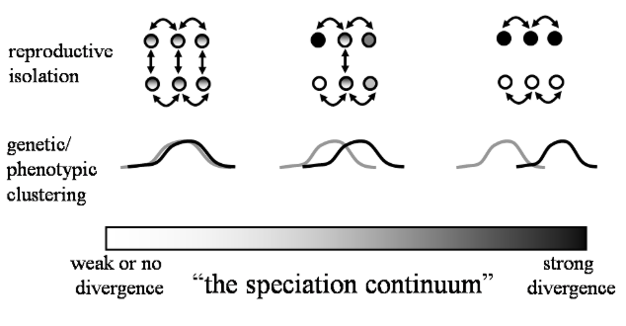
Under the commonly used ‘Biological Species Concept' (Mayr 1942), the formation of new species involves the evolution of reproductive barriers to the production of viable offspring either before (pre-zygotic barriers) or soon after (post-zygotic barriers) mating. Thus, new species form when individuals from diverging populations no longer recognize one another as potential mates, or opportunities for mating become limited by differences in habitat use or reproductive schedules. In some cases, these pre-zygotic isolating mechanisms fail to prevent inter-breeding among individuals from separate populations. In these cases, viable hybrids may form, or the consequences of a successful mating attempt may end in failure, either due to the production of inviable zygotes or sterile, non-reproductive offspring. These diverse pre- and post-zygotic barriers are of great importance to speciation biologists because they determine how reproductively-isolated populations are from one another, which indicates how far along the often continuous process of speciation that populations are. For example, reproductive isolation is weak in the early stages of speciation, but changes to strong or complete in later stages of speciation (Figure 2). One or more of the many types of isolating mechanisms may play a role in the evolution of species along a continuum (Figure 2). But how and why might reproductive barriers to genetic exchange evolve?
The role of geography in speciation.
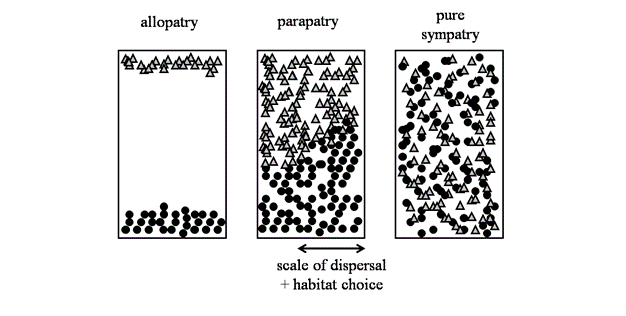
A major area of debate among speciation biologists is the geographic context in which it occurs (Figure 3). Ernst Mayr emphatically defended his view that speciation was most likely when populations became geographically isolated from one another, such that evolution within isolated populations would lead to enough differences among them that speciation would be an eventual outcome. "The ... evolution of isolating mechanisms as a by-product of the steady genetic divergence is inevitable" (Mayr 1963, p. 581). The central idea here is that when populations are geographically separated, they will diverge from one another, both in the way they look and genetically. These changes might occur by natural selection or by random chance (i.e., genetic drift), and in both cases result in reproductive isolation. This view of speciation of geographically isolated populations — termed allopatric speciation — is still widely held among speciation biologists as playing a major role in the evolution of biodiversity (e.g., Price 2007).However, speciation might also occur in overlapping populations that are not geographically isolated (i.e., sympatric speciation, Via 2001). The problem here is how do populations that are living in the same area, and exchanging genes, diverge from one another? Many biologists think this will be extremely difficult (Coyne & Orr 2004), but there are a few compelling examples where populations in different habitats are subject to contrasting patterns of natural selection (i.e., divergent selection) and overcome gene flow to diverge into different species. This could occur, for example, if insects adapted to living on different plants within the same geographic region (Feder et al. 1988). It will be interesting to see how many new examples emerge now that the idea of sympatric speciation is becoming less controversial. Another scenario for speciation in the face of gene flow, albeit at levels that are lower than during sympatric speciation, is ‘parapatric speciation'. Parapatric speciation refers to populations that are situated in geographic proximity to one another, usually with abutting but non-overlapping ranges. Here, a small proportion of each population are in actual contact with one another, and thus considered in sympatry, whereas the majority of individuals reside far enough apart that frequent encounters with one another are rare (Figure 3). There are putative examples of parapatric speciation in salamanders (Niemiller et al. 2008) and walking-stick insects (Nosil et al. 2002), but the phenomenon has received less attention that allopatric or sympatric speciation (Coyne & Orr 2004).
Mechanisms: Reclassification in the 1990s to Focus on Processes Driving Divergence
The 1990s saw a reclassification of modes of speciation away from schemes that focus solely on the geographic mode of divergence and towards a focus on the evolutionary process driving genetic divergence (i.e., the ‘mechanisms' of speciation). This reclassification was motivated — at least in part — by renewed interest in the extent to which the evolutionary processes which cause adaptation within species also tend to create new species. Further, although the geographic mode of divergence has important implications for speciation via patterns of gene flow and sources of selection, speciation research has reached the point where we can directly test the role of different evolutionary process in driving speciation (Butlin et al. 2008). We outline several processes that can drive speciation.
Natural selection and speciation: ‘Ecological speciation'.
Recent years have seen renewed efforts to address these questions. For example, populations living in different ecological environments (e.g., desert versus forest habitats) might undergo divergent and adaptive evolutionary change via divergent natural selection. These same evolutionary changes can also result in the populations evolving into separate species. For example, adaptation to different environments might cause differences between populations in the way individuals tend to look, smell, and behave. In turn, these differences might cause individuals from different populations to avoid mating with one another, or hybrids exhibit reduced fitness if mating occurs. Thus, the populations cease exchanging genes, thereby diverging into separate species because of the adaptive changes that occurred via natural selection. This is a simple description of the ‘ecological speciation' hypothesis (Rundle & Nosil 2005, Schluter 2009).
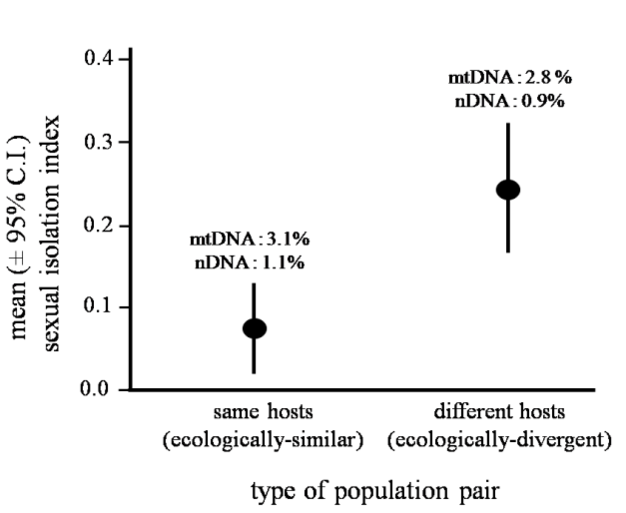
More specifically, ecological speciation is defined as the process by which barriers to gene flow evolve between populations as a result of ecologically-based divergent selection between environments. This process makes some simple predictions. For example, ecologically-divergent pairs of populations should exhibit greater reproductive isolation than ecologically-similar pairs of populations of similar age (Funk 1998). Figure 4 illustrates an example that supports this prediction. Other predictions are that traits involved in divergent adaptation will also cause reproductive isolation, and that levels of gene flow in nature will decrease as ecological differences between populations increase. All these predictions have now seen support, and outstanding questions concern the genetic bases of ecological speciation and the reasons why the process varies in how far it proceeds (Rundle & Nosil 2005).
The role of sexual selection in speciation.
A current debate is whether sexual selection can lead to speciation in the absence of ecological divergence (van Doorn et al. 2009). Indeed, compelling examples that implicate an important role of sexual selection leading to new species sometimes also involve the evolution of different signals used in mate-selection among populations in different ecological contexts, such as light environment (Seehausen et al. 2008, Maan & Seehausen 2010). Here, signals used in mate-selection become adapted to new ecological environments where the transmission of these traits is more perceptible or audible in a new habitat.
Random processes/drift.
Another mechanism of speciation that involves chance events is speciation by polyploidization. Polyploidy, or the presence of three or more complete sets of chromosomes, has been documented in a wide variety of taxa. Particularly prevalent in plants, between 47 and 70% of all angiosperms are polyploid (Ramsey & Schemske 1998). Because polyploidy can lead to hybrid infertility, it is viewed as a mechanism that can rapidly lead to the formation of new species, potentially without selection for the divergence of other characters.
Current views: Mutation-order vs. ecological speciation.
Genetics/Genomics: New Directions with Genetics
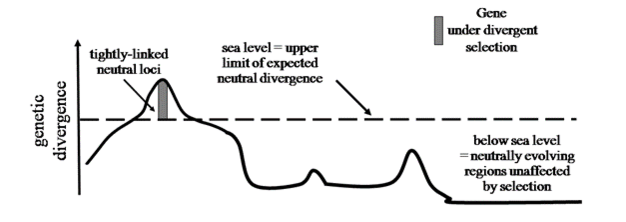
Genetic studies have long-been at the forefront of speciation research (Coyne & Orr 2004). For example, studies examining the genetic basis of hybrid sterility and inviability have supported the existence of ‘Dobzhansky-Muller Incompatibities' and patterns predicted by ‘Haldane's Rule'. Recent advances in genomics now allow such studies to be taken to the genome-wide level, where biologists can examine hundreds of thousands of gene regions, rather than just a handful. To help understand this genome-wide variation, biologists have developed the metaphor of ‘genomic islands of divergence' (Turner et al. 2005). A genomic island is any gene region, be it a single nucleotide or an entire chromosome, which exhibits significantly greater differentiation than expected under neutrality (i.e., divergence by genetic drift alone). The metaphor thus draws parallels between genetic differentiation observed along a chromosome and the topography of oceanic islands and the contiguous sea floor through which they are connected. Following this metaphor, sea level represents the threshold above which observed differentiation is significantly greater than expected by neutral evolution alone. Thus, an island is composed of both directly selected and tightly linked loci. Major remaining questions concern the size, number and distribution (i.e., chromosomal location) of these genomic islands, and how variation in these factors affects the process of speciation. Clear answers to these questions will likely require experimental studies that measure selection at the genomic level to directly quantify how selection acts on the genome. Nevertheless, the integration of geographic, ecological, and new genomic approaches is likely to yield new insight into speciation over the coming decades.
Glossary
Divergent natural selection: Selection that acts in contrasting directions between two populations, usually with reference to ecological differences between their environments (e.g., large body size confers high survival in one environment and low survival in the other), or the special case where selection favors opposite extremes of a trait within a single population (i.e., disruptive selection).
Ecological speciation: A speciation process in which divergent natural selection drives the evolution of reproductive incompatibility (i.e., isolation) between taxa.
Mutation-order speciation: A speciation process in which different and incompatible mutations (alleles) fix in separate populations that are experiencing similar selective regimes.
Dobzhansky-Muller Incompatibility: Hybrid dysfunction arising from negative interactions (epistasis) between alleles at two or more loci: an allelic substitution at a locus causes no reduction in fitness on its own genetic background, but leads to reduced fitness when placed on the alternative background.
Haldane's Rule: The observation that if only one sex of hybrid offspring suffers intrinsic sterility or inviability, it is the heterogametic sex (see Coyne & Orr 2004 for a review of the causes of this pattern).
Genomic Island: A region of the genome where differentiation between populations is stronger than expected in the absence of divergent selection (stronger than occurs via purely neutral processes such as genetic drift alone).
Natural selection: Differential survival of classes of entities (such as alleles) which differ in some characteristic(s).
Sexual selection: Differential reproductive success of classes of entities (such as alleles) which differ in some characteristic(s).
Reproductive Isolation: Genetically-based differences between populations which reduce or prevent genetic exchange between them (i.e., reproductive barriers).
References and Recommended Reading
Coyne, J. A. & Orr, H. A. Speciation. Sunderland, MA: Sinauer Associates, 2004.
Darwin, C. On The Origin of Species by Means of Natural Selection, or The Preservation of Favoured Races in the Struggle for Life. London, UK: John Murray, 1859. (link)
Feder, J. L., Chilcote, C. A. & Bush, G. L. Genetic differentiation between sympatric host races of Rhagoletis pomonella. Nature 336, 61–64 (1988).
Funk, D. J. Isolating a role for natural selection in speciation: Host adaptation and sexual isolation in Neochlamisus bebbianae leaf beetles. Evolution 52, 1744–1759 (1998).
Maan, M. E. & Seehausen, O. Mechanisms of species divergence through visual adaptation and sexual selection: Perspectives from a cichlid model system. Current Zoology 56, 285–299 (2010).
Mallet, J. et al. Space, sympatry and speciation. Journal of Evolutionary Biology 22, 2332–2341 (2009).
Mani, G. S. & Clarke, B. C. Mutation order — A major stochastic process in evolution. Proceedings of the Royal Society B: Biological Sciences 240, 29–37 (1990).
Mayr, E. Systematics and the Origin of Species. New York, NY: Columbia University Press, 1942.
Mayr, E. Animal Species and Evolution. Harvard, MA: Harvard University Press, 1963.
Mayr, E. & Provine, W. B. The Evolutionary Synthesis. Harvard, MA: Harvard University Press, 1998.
Niemiller, M. R., Fitzpatrick, B. M. & Miller, B. T. Recent divergence with gene flow in Tennessee cave salamanders (Plethodontidae: Gyrinophilus) inferred from gene genealogies. Molecular Ecology 17, 2258–2275 (2008).
Nosil, P., Crespi, B. J. & Sandoval, C. P. Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417, 440–443 (2002).
Nosil, P., Harmon, L. J. & Seehausen, O. Ecological explanations for (incomplete) speciation. Trends in Ecology & Evolution 24, 145–156 (2009).
Nosil, P., Funk, D. J. & Ortíz-Barrientos, D. Divergent selection and heterogeneous genomic divergence. Molecular Ecology 18, 375–402 (2009).
Panhuis, T. M. et al. Sexual selection and speciation. Trends in Ecology & Evolution 16, 364–371 (2001).
Price, T. D. Speciation in Birds. Woodbury, NY: Roberts and Company, 2007.
Ramsey, J. & Schemske, D. W. Pathways, mechanisms and rates of polyploid formation in flowering plants. Annual Review of Ecology, Evolution, and Systematics 29, 467–501 (1998).
Ritchie, M. G. Sexual selection and speciation. Annual Review of Ecology, Evolution, and Systematics 38, 79–102 (2007).
Rundle, H. D. & Nosil, P. Ecological speciation. Ecology Letters 8, 336–352 (2005).
Schluter, D. Ecology and the origin of species. Trends in Ecology & Evolution 16, 372–380 (2001).
Schluter, D. Evidence for ecological speciation and its alternative. Science 323, 737–741 (2009).
Seehausen, O. et al. Speciation through sensory drive in cichlid fish. Nature 455, 620–626 (2008).
Turner, T. L., Hahn, M. W. & Nuzhdin, S. V. Genomic islands of speciation in Anopheles gambiae. PLoS Biology 3, e285 (2005). doi:10.1371/journal.pbio.0030285
van Doorn, S., Edelaar, P. & Weissing, F. J. On the origin of species by natural and sexual selection. Science 326, 1704–1707 (2009).
Via, S. Sympatric speciation in animals: The ugly duckling grows up. Trends in Ecology & Evolution 16, 381–390 (2001).