« Prev Next »
How Sexual Selection Came To Be Recognized
Charles Darwin proposed that all living species were derived from common ancestors. The primary mechanism he proposed to explain this fact was natural selection: that is, that organisms better adapted to their environment would benefit from higher rates of survival than those less well equipped to do so. However he noted that there were many examples of elaborate, and apparently non-adaptive, sexual traits that would clearly not aid in the survival of their bearers. He suggested that such traits might evolve if they are sexually selected, that is if they increase the individual's reproductive success, even at the expense of their survival (Darwin 1871).
Darwin noted that sexual selection depends on the struggle between males to access females. He recognized two mechanisms of sexual selection: intrasexual selection, or competition between members of the same sex (usually males) for access to mates, and intersexual selection, where members of one sex (usually females) choose members of the opposite sex. The idea of cumbersome traits evolving to aid males in competition during aggressive encounters was readily accepted by scientists shortly after Darwin's publication. However, the idea of female mate choice was received with ridicule, and was not seriously reconsidered until nearly 80 years later (Cronin 1991). In the 40 years since, there has been much progress in our understanding of how sexual selection operates.
Which Sex is Under Stronger Selection?
Sex roles are defined by differences in gametes: females produce relatively few, highly nutritious (usually non-motile) gametes, whereas males produce comparatively abundant, smaller, motile gametes. Because only a single gamete of each type is required to produce an offspring, there will be an excess of male gametes that will not fertilize any eggs. This asymmetry leads to Bateman's principle, whereby female reproduction is primarily limited by their access to resources to nourish and produce these large gametes, whereas male reproduction is mainly limited by access to females (Bateman 1948). Therefore males typically compete among themselves for access to females, whereas females tend to be choosy and mate only with preferred males.
In sexually reproducing species, every offspring has one father and one mother, so the average reproductive success is equal for both males and females. A successful male can potentially sire many offspring. If a male gains a disproportionate share of reproduction, he will take away reproductive opportunities from other males, leading to a high reproductive variance among males. A successful female, on the other hand, will not take away reproductive opportunities from other females, leading to a smaller variance in reproductive success. The higher the reproductive variance, the stronger the effects of sexual selection (Figure 1). Strong sexual selection typically results in sexually dimorphic traits that are exaggerated, or more elaborate, in the sex with highest reproductive variance (Figure 1).
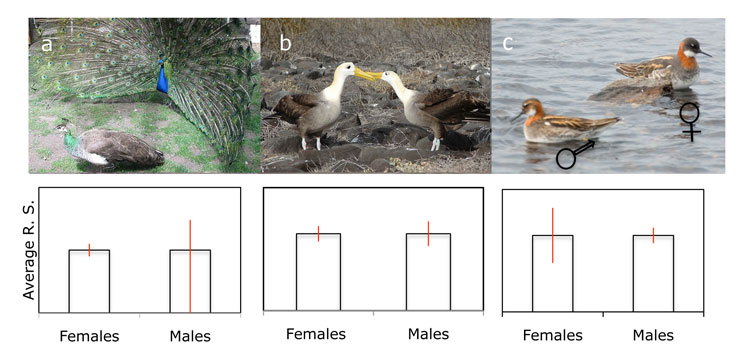
The Role of Parental Care
How Does Sexual Selection Operate?
Choosing a Mate
Good Genes
Under the ‘good genes’ scenario, differences among males provide females with information about the genetic qualities of the different males that can be inherited by the offspring. Under the ‘good genes,’ just as in the ‘direct benefits’ models, there is correspondence between the putative roles of natural versus sexual selection, since preferring certain males can result in a female gaining higher viability, fecundity, and reproductive success, for her offspring. Good genes can be those that allow males to carry a ‘handicap,’ yet survive despite having a cumbersome trait (Zahavi 1975), genes that signal resistance to disease (Hamilton & Zuk 1982), or genes that are more compatible with those of the female (Trivers 1972). Evidence of female choice for good genes remains scarce despite decades of studies of female mate choice in many taxa. This apparent lack of success continues to create debate as to the importance of the good genes model in the field.
Fisherian Arbitrary Choice
When Does Sexual Selection Act?
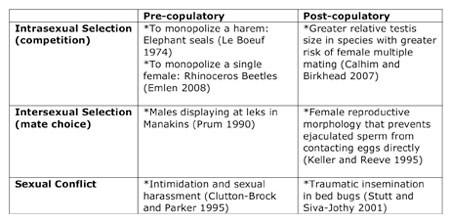
Sexual selection can affect reproductive success at multiple reproductive stages. First, it acts during all the processes that lead to acquiring mating opportunities (i.e., excluding competitors, attracting, selecting and/or retaining mates). Darwin referred exclusively to pre-copulatory sexual selection in his discussions, erroneously assuming that mating would inevitably result in reproductive success. In recent years, evidence that copulatory and post-copulatory events play an important role in determining the outcome of fertilization and reproduction has been increasing. Post-copulatory selection refers to the events that occur during and after mating. Post-copulatory male-male competition is known as sperm competition a term coined by Parker (1970) who recognized that when females mate with multiple males, their ejaculates compete inside the female reproductive tract for access to eggs. Sperm competition has resulted in the evolution of morphologically modified sperm that increase the likelihood of fertilization in many taxa (Birkhead & Moller 1998). Post-copulatory female choice refers to the ability of females to affect the likelihood that sperm from a particular male fertilizes their eggs, and their decision to invest in offspring based on the identity of the male with whom they mate. Females exert this choice via morphological, chemical and behavioral adaptations. This type of selection is called cryptic choice because it occurs inside the female reproductive tract and cannot be detected from behavioral studies alone (Eberhard 1996).
Conflict Between the Sexes
Although both sexes are seeking to optimize their reproductive success, their genetic interests are not aligned, resulting in sexual conflict (Parker 1979). Traits that allow a male to increase his reproductive success at the expense of the female will be positively selected if the female mates with multiple males. These traits will be genetically transmitted and spread in the population, despite their negative effects on female reproductive success, if the reproductive success of these males is higher than that of males lacking such traits (Parker 1979). Sexual conflict can often result in an evolutionary arms race, whereby the evolution of a trait that imposes harm on one sex will result in evolution of a counter-trait to mitigate the harm on the affected sex, with subsequent escalation in both (Chapman et al. 2003). Examples of sexual conflict include traumatic insemination in bed bugs, copulatory grasping and anti-grasping structures in waterstriders, and genital coevolution in waterfowl.
References and Recommended Reading
Bateman, A. J. Inter-sexual selection in Drosophila. Heredity 2, 349-368 (1948).
Birkhead, T. R. & Moller, A. P. Sperm Competition and Sexual Selection. San Diego, CA: Academic Press, 1998.
Calhim, S. & Birkhead, T. R. Testes size in birds: quality versus quantity — assumptions, errors and estimates. Behavioral Ecology 18, 271-275 (2007).
Chapman, T., Arnqvist, G. et al. Sexual conflict. Trends in Ecology and Evolution 3, 41-47 (2003).
Clutton-Brock, T. H. & Parker, G. A. Sexual coercion in animal societies. Animal Behavior 49, 1345-1365 (1995).
Cronin, H. The Ant and the Peacock. Cambridge, UK: Cambridge University Press, 1991.
Darwin, C. The Descent of Man, and Selection in Relation to Sex. London, UK: Murray, 1871.
Eberhard, W. Female Control: Sexual Selection by Cryptic Female Choice. Princeton, NJ: Princeton University Press, 1996.
Emlen, D. J. The Evolution of Animal Weapons. Annual Review of Ecology, Systematics, and Evolution 39, 387-413 (2008).
Fisher, R. A. The Genetical Theory of Natural Selection. Oxford, UK: Clarendon Press, 1930.
Hamilton, W. D. & Zuk, M. Heritable true fitness and bright birds: a role for parasites? Science 218, 384-387 (1982).
Keller, L. & Reeve, H. K. Why do females mate with multiple males? The sexually selected sperm hypothesis. Advanced Studies in Behavior, 24, 291-315 (1997).
Kirkpatrick, M. Sexual selection and the evolution of female choice. Evolution 82, 1-12 (1982).
Lande, R. Models of speciation by sexual selection on polygenic traits. Proceedings of the National Academy of Sciences, USA 78, 3721-3725 (1981).
LeBoeuf, B. Male-male competition and reproductive success in Elephant seals. American Zoologist 14, 163- 176 (1974)
Parker, G. Sperm competition and its evolutionary consequences in the insects. Biological Reviews 45, 525-567 (1970).
Parker, G. Sexual selection and sexual conflict. In Sexual Selection and Reproductive Competition in Insects. eds. Blum, M. S. & Blum, N. A. (New York: Academic Press, 1979): 123-166.
Prum, R. O. Phylogenetic analysis of the evolution of display behavior in the neotropical manakins (Aves: Pipridae). Ethology 84, 202-231 (1990).
Stutt, A. D., Siva-Jothy, M. T. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proceedings of the National Academy of Sciences, U.S.A., 98, 5683-5687 (2001)
Trivers, R. L. Parental investment and sexual selection. In Sexual Selection and the Descent of Man 1871-1971. ed. Campbell, B. (London: Heinemann 1972): 136-179.
Zahavi, A. Mate selection: a selection for a handicap. Journal of Theoretical Biology 53, 205-214 (1975).































