« Prev Next »
Introduction
Secondary production (or the formation of heterotrophic biomass through time) is a topic often considered as a small part of the energy-flow paradigm commonly described in most ecology texts. The energy-flow paradigm begins with energy from the sun being converted into autotrophic biomass by photosynthesis. This formation of biomass (primary production) is ultimately passed on to successive heterotrophic levels via ingestion. Because heterotrophs such as animals do not assimilate all their food and have substantial respiratory demands, only about 10% of what they ingest is converted into their own secondary production according to the paradigm. Energy flow diagrams often depict secondary production as the flow leaving one trophic level and entering (being ingested by) the next. Many ecologists, however, have demonstrated that secondary production, particularly when measured at the population level, has far wider usefulness than solely in describing the flow of energy between trophic levels. Applications extend to questions on single species (habitat-specific microdistributions), communities (e.g., niche overlap and competition), and ecosystems (chemical flows and stoichiometry) (Benke 2010a, Benke & Huryn 2010).
One application of secondary production is in the quantification of food webs. Many food webs described in the ecological literature are "connectivity" webs, in which species are depicted as nodes (dots) and interactions (or linkages) among species are shown as lines connecting the nodes. Such webs have sometimes suggested great complexity in the many linkages between species, but this approach has numerous shortcomings such as not identifying the relative importance of species and not quantifying their interactions (Woodward et al. 2005). As a result there have been attempts to quantify foods webs by measuring various attributes of their interactions (e.g., Berlow et al. 2004, Woodward et al. 2005). One such attempt is determination of taxon-specific secondary production within the assemblage which can lead to measurement of energy or material flows among species (Benke & Wallace 1980, 1997). The key feature of this approach is combining production with diet analyses of those same taxa in order to build detailed quantitative flow webs. Such flow webs represent a significant departure from the early energy flow paradigm described above in terms of complexity and methodology. We might think of flow webs as a type of food web that combines the quantitative aspects of trophic-level energy flow with the species-level detail often observed in connectivity webs (e.g., Closs & Lake 1994, Woodward 2005). The units of such flow webs are typically mass or energy per m2 per unit time (e.g., grams m-2 y-1). Ingestion flows from a single resource (or prey species), for example, may be distributed to many consumers (or predators) and individual flows throughout the community may vary by several orders of magnitude. Such quantitative differences in ingestion flows can thus serve as a measure of bottom-up interaction (or linkage) strength between species and their food resource (Benke & Wallace 1997). That is, the strength of any interaction in the web is measured by the absolute amount of food ingested by each consumer.
Other aspects of food webs may be derived from the basic flow web. The ratio of these same ingestion flows to production of the resource (or prey) from which they came (ingestion/production) may be used as a top-down measure of interaction strength or predation pressure (Benke et al. 2001, Woodward et al. 2005). That is, if a consumer (predator) ingests a large fraction of a resource's (prey's) production (regardless of the absolute amount of that production), it would imply a strong top-down interaction. Finally, the combination of ingestion flows to any species in a flow web can be used to calculate the trophic position of that species (Levine 1980, Hall et al. 2000; Benke et al. 2001). Trophic position, rather than trophic level, shows exactly where a species fits into the trophic hierarchy (e.g., species A has a trophic position of 3.2 rather than being placed within the third trophic level of all secondary consumers). This is exactly the same concept that has been addressed using stable isotopes (e.g., Vander Zanden & Rasmussen 1999; Post 2002; McCutchan et al. 2003).
The general application of secondary production in building flow webs depends heavily on our ability to measure production in the field. Fortunately, production methods have been well established in all environments (freshwater, marine, terrestrial) since at least the 1960s. In recent decades, however, secondary production has been most widely studied for freshwater and marine benthic invertebrates (Benke 1984, Cusson & Bourget 2005, Benke & Huryn 2010), so it is here that we turn for examples of quantitative food webs. In stream ecosystems, numerous investigators have successfully determined taxon-specific production for entire invertebrate assemblages (e.g., Benke et al. 1984, Wallace et al. 1999, Hall et al. 2000). Detailed quantitative gut analyses have sometimes been accomplished in stream assemblages as well (e.g., Wallace et al. 1987, Woodward et al. 2005). Combining assemblage-wide production estimates with gut analyses, and assuming food-specific ecological efficiencies (e.g., 0.40 of algae ingested and 0.70 of animals ingested are assimilated from the digestive tract) allows one to determine the trophic basis of production (Benke & Wallace 1980). This tells us how much each food source is responsible for the production of each species. Subsequently, flow webs can be built using all species, or at least the common species, in the assemblage (e.g., Benke & Wallace 1997, Benke et al. 2001; Hall et al. 2000).
The objectives of this article are to:
- describe a hierarchy of food webs, beginning with a simple connectivity web, by identifying the presence or absence of food items through gut analysis,
- convert the connectivity web into a diet proportion web by quantification of gut analyses,
- convert the diet proportion web into an assimilation web using ecological efficiencies,
- convert the assimilation web into a quantitative flow web using secondary production,
- convert the flow web into an ingestion/production web, and
- determine trophic position for each species in the flow web.
Objectives 3–6 are based on energetic principles involving ecological efficiencies, production analyses, or both. To accomplish these objectives, I use a fictional food web that has been simplified from the more complex web of a riverine invertebrates found on submerged wood (Benke & Wallace 1997; Benke et al. 2001). As each type of food web is constructed, increasing amounts of information are required. It should be emphasized that the food webs shown here are not the only approaches used in attempting to quantify food web interactions.
Types of Food Webs and How to Construct Them
Connectivity webs may be constructed with qualitative gut content information without regard to relative proportions in the gut. Such webs may be built from gut presence/absence data or from literature information. Many investigators have used connectivity webs to calculate various statistics such as the number of interactions per species, maximum food chain length from basal resources to top predator, etc. (Woodward et al. 2005). Although early versions of this approach were criticized for their simplicity and poor quality data, there are now excellent examples of highly detailed webs for streams from gut analyses (e.g., Closs & Lake 1994; Woodward et al. 2005). The major remaining weakness of this approach, no matter how detailed the web, is that it does not account for different linkage strengths between species, however we might measure strength (Benke & Wallace 1997).
Diet proportion webs are based on quantitative gut analyses such as with methods described by Wallace et al. (1987) or Woodward et al. (2005) for stream invertebrates. They represent the simplest type of quantification beyond the connectivity web, and show to what extent a given consumer eats different fractions of its food resources. Tavares-Cromar & Williams (1996) built some very detailed stream webs using this approach. Such webs are more informative than a connectivity web, and line thicknesses from each food resource to a consumer can be used to represent the fraction of each food item ingested. Thus, in a food web diagram, all lines entering a given consumer should sum to 1.00. While line thicknesses reflect dietary preferences for each consumer, differences in line thicknesses between consumer species have little meaning because they do not reflect differences in absolute ingestion.
Assimilation webs add an additional level of quantification beyond the diet proportion web. Assimilation is that portion of ingested food that is assimilated (absorbed) from the digestrive tract for use in metabolism and growth. Assimilation efficiencies (assimilation/ingestion, or "A/I" efficiencies) vary greatly depending on the type of food ingested (e.g., animal food v. detritus). The relative amount of each food type assimilated (and thus contributing to production) can be determined by multiplying the diet proportion of a food type by its assimilation efficiency; that is, you are what you assimilate rather than you are what you ingest. The relative amount assimilated is easily converted into the fractional amount assimilated. Differences between the diet proportion web and the assimilation web are often subtle and only apparent for animals that feed on a diverse diet (i.e., animals ingesting foods having different assimilation efficiencies). Again, line thicknesses only apply to fractional amounts assimilated by a species, all of which sum to 1.00. Although I am unaware of anyone constructing an assimilation web, the information from such a web can be combined with secondary production to determine the trophic basis of production; that is., how much secondary production of a single consumer species is attributed to each food resource (Benke & Wallace (1980). Total production of all species attributable to a single food resource can also be determined. Several studies have now determined the trophic basis of production for groups of similar taxa (e.g., Roeding & Smock 1989; Benke & Jacobi 1994) and entire communities (e.g., Smock & Roeding 1986; Hall et al. 2001).
Flow webs represent a step beyond the assimilation web and trophic basis of production. The absolute ingestion flow between a specific food resource and its consumer species is estimated from the production attributable to the food resource divided by gross production efficiency (Benke & Wallace 1997). Gross production efficiency is assimilation efficiency (A/I) times net production efficiency, the latter being production divided by assimilation (P/A). Thus, GPE = A/I x P/A = P/I. Because GPE is a fraction considerably less than one, ingestion of a food resource will be substantially higher than the production it creates. Once individual ingestion flows (g m-2 y-1) are determined for each interaction, a quantitative flow web can be constructed for the entire assemblage/community. Many flow webs have now been built in this manner, primarily in stream systems (e.g., Hall et al. 2000, Benke et al. 2001, Cross et al. 2007, Runck 2007). Flow webs demonstrate major differences in the quantity of materials or energy flow pathways within food webs; these can vary by orders of magnitude within communities or in comparisons among communities. This is the only food web described here that demonstrates absolute differences in ingestion flows among consumers.
Once a quantitative flow web has been constructed, trophic position can be determined for individual species (Levine 1980, Hall et al. 2000, Benke et al. 2001). Although the trophic level of a consumer can be determined by following the longest feeding chain, trophic position for that same consumer can be calculated as 1 plus the sum of the trophic position of each food item times the fraction each food item contributed to the consumer's production (as in the trophic basis of production). For example, TP = 1 + (2 x 0.9) + (2.8 x 0.10) = 3.1, where TP is trophic position of a consumer species, 2 and 2.8 are trophic positions of 2 food types ingested by that species and 0.9 and 0.1 are the fractions these food types contributed to production of the species. If one wants to determine the trophic position for all species, it is necessary to determine the trophic position for those species closest to the basal resources first.
Ingestion/production webs represent an attempt to quantify the top-down effect of a predator on its prey (or food resource). Each interaction is measured as the fraction (I/P) of a prey species' production (P) that is ingested (I) by one of its predators. For example, if production of a prey species is 10 g m-2 y-1 and one of its predators consumes 1 g m-2 y-1 of its production, then the prey's I/P value for this predator is 0.10. Because both I and P have the same units (g m-2 y-1), this ratio in unitless. The sum of fractions of a prey species to all its predators represents the total fraction of production that is ingested and the total impact of all predators on this species (Benke et al. 2001, Woodward et al. 2005). Thus, while any one predator may have a weak interaction with a given prey (e.g., 0.10), this approach shows that the sum of, say, 9 similar predators, each with P/I = 0.10, may represent a large impact (i.e., 0.90) on the prey. The sum of all such top-down interactions for a given prey species thus has a range from zero to 1.00.
Comparisons of Food Webs Constructed with the Same Data
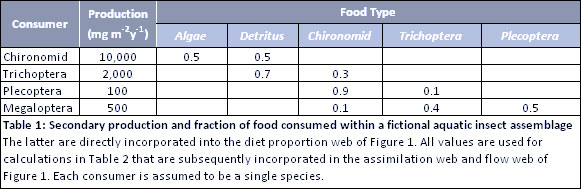
The fictional stream food web consists of two basal food resources (algae and detritus) and four species of stream insects: a chironomid midge, an omnivorous trichopteran (caddisfly), a predaceous plecopteran (stonefly), and a predaceous megalopteran (hellgrammite) (Table 1).

The diet proportion web of Figure 1 is based on the feeding fractions from Table 1 rather than just presence-absence data of the connectivity web. The lines from the food sources have different thicknesses corresponding to their fraction in the gut. The total of all lines entering each consumer must sum to 1.00. For example, the diet proportion web shows that 0.50 of the chironomid food is algae and 0.50 is detritus. It shows that 0.70 of trichopteran food is detritus and 0.30 is chironomid.
The assimilation web appears very similar to the diet proportion web except for animals (chironomids and trichopterans) that feed on food types having different assimilation efficiencies (Figure 1, Table 2). All lines entering each consumer again sum to 1.00 but are based on the fraction that each contributes to production (i.e., fraction that is assimilated). While only 0.30 of trichopteran ingestion is chironomids (see diet proportion web), the high assimilation efficiency (0.70) of chironomids results in chironomids being responsible for 0.75 of trichopteran production (i.e., of the food assimilated, 0.75 is from chironomids). Also, while chironomids eat equal amounts of algae and detritus (see diet proportion web), they assimilate much more of the algae (0.80) in the assimilation web.
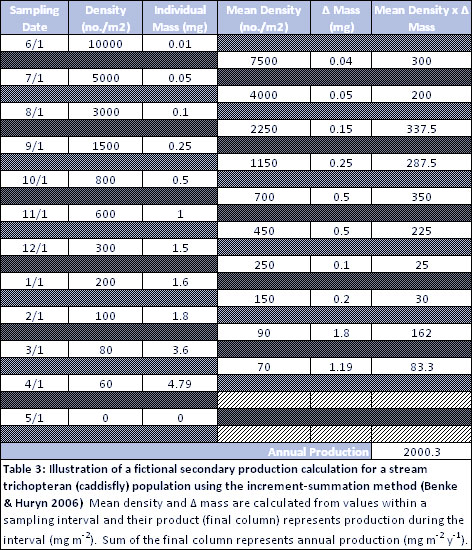
The flow web shows the actual amount of food ingested after taking production and ecological efficiencies into account (Figure 1, Tables 1, 2). It shows great variation among ingestion flows ranging from 29 to 40000 mg m-2 y-1 (last column of Table 2) as illustrated by line thickness in Figure 1. The wide range in flows is largely explained by great variation in production among species. Because the flow web is highly dependent on production of each consumer, an illustration of the production calculation for Trichoptera is shown in Table 3. The annual production summary of all 4 species is found in Table 1. Ingestion flows are obviously highest for those with highest production (Figure 1, Tables 1, 2). Chironomids ingest the greatest amounts of both algae (40,000 mg m-2 y-1) and detritus (40,000 mg m-2 y-1) in this assemblage. This equality of ingestion may seem surprising since 0.80 of chironomid production is due to eating algae (see assimilation web), the result of differing assimilation efficiencies. It may also seem surprising that chironomids consume more detritus than is consumed by trichopterans, even though the diet proportion web shows that trichopterans have more detritus in their guts (0.70) than is found in chironomid guts (0.50). Finally, note that the omnivorous trichopteran in the flow web consumes far more animal prey (4286 mg m-2 y-1) than either of the species considered as strict predators (286 mg m-2 y-1 for the plecopteran and 1428 mg m-2 y-1 for the megalopteran, last column of Table 2), which is a direct function of differences in production and total ingestion.
The ingestion/production (I/P) web shown on the right side of Figure 1 is constructed from data in Table 4 which summarizes production of each prey species, ingestion flow to each consumer, I/P for each individual flow and total I/P for each food source. Flows for production and ingestion were derived from Tables 1 and 2, respectively. Values also have been added for net primary production of algae and annual inflow of detritus to make the complete I/P web possible (Table 4). In this fictional web, individual impacts (I/P values in parentheses, Table 4) of predator species on prey species (or resources) vary widely from 0.01 to 0.80, but total predatory impacts on a given prey species range from only 0.37 to 0.80 (in parentheses, last column). Clearly the highest impacts for species-species interactions are imposed by chironomids on both algae (0.80) and detritus (0.50), Trichoptera on chironomids (0.43), Megaloptera on Trichoptera (0.36), and Megaloptera on Plecoptera (0.57).
Trophic position is calculated from the flow web (Figure 1) and is shown along with trophic level in Figure 2. There are 5 trophic levels in this simple system if one follows the longest feeding chain from either algae (level 1) or detritus (1) to chironomids (2) to Trichoptera (3) to Plecoptera (4) to Megaloptera (5). Both algae and detritus are basal resources and considered to be at trophic level 1 and trophic position 1. Similarly, chironomids are at trophic level 2 and trophic position 2. Trichopterans, however, obtain their food from more than one trophic position and while they are at trophic level 3, their trophic position is only 2.8. Subsequently, the level-4 plecopterans are at trophic position 3.1 and the level-5 megalopterans are at trophic position 3.4.
Discussion

The flow web is probably the most informative of all webs considered because it represents actual flows (rather than ratios) which have meaning across species and communities (e.g., ingestion of chironomids primarily passes through the omnivorous trichopteran far more than through the predaceous plecopteran or megalopteran) (Figure 1, Table 2). The flow web, and the individual flows that comprise it, can be considered as one way to quantify interaction (or linkage) strength from a bottom-up perspective throughout the assemblage.
If one interprets interaction strength from a top-down perspective (as many do), however, this can be accomplished with the I/P web (e.g., Woodward et al. 2005). For example, the trichopteran ingestion of 0.43 chironomid production suggests a relatively strong linkage (Figure 1). This is not the entire impact on chironomids because two other predators consume them as well, increasing their total I/P to 0.52. In the case of the chironomid-Trichoptera linkage both the flow web and I/P web indicate a strong interaction. Comparisons between other flow web and I/P web links indicate exactly the opposite relationship. Thus, there is no predictable pattern between the flow web and I/P web, as has been pointed out by Paine (1980) and others. For real stream food webs, however, total ingestion by predators has sometimes been shown to be >90% of prey production (e.g., Smith & Smock 1992, Wallace et al. 1997, Huryn 1998). Such high total I/P suggests a strong top-down influence, but it may be distributed among several predators with individual linkage strengths well below 1.00 (diffuse predation).
Trophic position is an interesting concept because it recognizes that many consumer species do not easily fall into trophic levels but rather occur in a staggered hierarchy. For example, the megalopteran could be identified at the fifth trophic level but is actually only at trophic position 3.4 because some of its prey are at trophic positions <3 (Figure 2). Results using this flow web approach should be very similar to trophic positions identified by stable isotope (SI) methods. But both methods depend on the accuracy of certain assumptions. For example, the SI approach assumes a constant fractionation from one trophic level to the next of 3.4. The flow web approach assumes that the selected assimilation efficiencies are reasonable approximations of reality. A major advantage of SI methods for estimating trophic position is that measurement is much less time-consuming than the flow web and it can be done for individual species of interest. On the other hand, the SI approach cannot itself define a true food web because linkages between species are not identified. It would be enlightening to compare these approaches for calculating trophic position in a single system, but I am unaware of any attempts to do so.
In summary, among the many ways in which secondary production has been used in addressing ecological questions (Benke & Huryn 2010) is its application to food web analysis. The same assemblage-level production information enables the quantification of at least three food-web approaches in contemporary community ecology: bottom-up linkages with flow webs, top-down linkages with ingestion/production webs, and assessment of trophic positions.
References and Recommended Reading
Benke, A. C. "Secondary production of aquatic insects." In Ecology of Aquatic Insects, eds. V. H. Resh & D. M. Rosenberg (New York, NY: Praeger Publishers, 1984): 289–322.
———. Concepts and patterns of invertebrate production in running waters. Verhandlungen der internationale Vereinigung für theoretische und angewandte Limnologie 25, 15–38 (1993).
———. Secondary production as part of bioenergetic theory - contributions from freshwater benthic science. River Research and Applications 26, 36–44 (2010a).
———. Secondary Production. Nature Education Knowledge 1, 5 (2010b).
Benke, A. C. & Huryn, A. D. "Secondary production of macroinvertebrates," In Methods in Stream Ecology, 2nd ed., eds. F. R. Hauer & G. A. Lamberti (Burlington, MA: Academic Press, 2006): 691–710.
———. Benthic invertebrate production: facilitating answers to ecological riddles in freshwater ecosystems. Journal of the North American Benthological Society 29, 264–285 (2010).
Benke, A. C. & Jacobi, D. I. Production dynamics and resource utilization of snag-dwelling mayflies in a blackwater river. Ecology 75, 1219–1232 (1994).
Benke, A. C. & Wallace, J. B. Trophic basis of production among net-spinning caddisflies in a southern Appalachian stream. Ecology 61, 108–118 (1980).
———. Trophic basis of production among riverine caddisflies: implications for food web analysis. Ecology 78, 1132–1145 (1997).
Benke, A. C. et al. Food web quantification using secondary production analysis: predaceous invertebrates of the snag habitat in a subtropical river. Freshwater Biology 46, 329–346 (2001).
Closs, G. P. & Lake, P. S. Spatial and temporal variation in the structure of an intermittent-stream food web. Ecological Monographs 64, 1–21 (1994).
Cross, W. F. et al. Nutrient enrichment reduces constraints on material flows in a detritus-based food web. Ecology 87, 1556–1565 (2007).
Cusson, M. & Bourget, E. Global patterns of macroinvertebrate production in marine benthic habitats. Marine Ecology Progress Series 297, 1–14 (2005).
Hall, R. O. et al. Organic matter flow in stream food webs with reduced detrital resource base. Ecology 81, 3445–3463 (2000).
Hall, R. O. et al. Trophic basis of invertebrate production in 2 streams at the Hubbard Brook Experimental Forest. Journal of the North American Benthological Society 20, 423–447 (2001).
Huryn, A. D. Ecosystem-level evidence for top-down and bottom-up control of production in a grassland stream system. Oecologia (Berlin) 115, 173–183 (1998).
Levine, S. Several measures of trophic structure applicable to complex food webs. Journal of Theoretical Biology 83, 195–207 (1980).
McCutchan, Jr., J. H. et al. . Oikos 102, 378–390 (2003).
Paine, R. T. Foodwebs: linkage, interaction strength and community infrastructure: the third Tansley lecture. Journal of Animal Ecology 49, 667–685 (1980).
Post, D. M. Using stable isotopes to estimate trophic position: models, methods, assumptions. Ecology 83, 703–718 (2002).
Roeding, C. E. & Smock, L. A. Ecology of macroinvertebrate shredders in a low-gradient sandy-bottomed stream. Journal of the North American Benthological Society 8, 149–161 (1989).
Runck, C. Macroinvertebrate production and food web energetics in an industrially contaminated sream. Ecological Applications 17, 740–753 (2007).
Smock, L. A. & Roeding, C. E. The trophic basis of production of the macroinvertebrate community of a southeastern USA blackwater stream. Holarctic Ecology 9, 165–174 (1986).
Tavares-Cromar, A. F. & Williams, D. D. The importance of temporal resolution in food web analysis: evidence from a detritus-based stream. Ecological Monographs 66, 91–113 (1996).
Vander Zanden, M. J. & Rasmussen, J. B. Primary consumer d13C and d15N and the trophic position of aquatic consumers. Ecology 80, 1395–1404 (1999).
Wallace, J. B. et al. Trophic pathways of macroinvertebrate primary consumers in subtropical blackwater streams. Archiv für Hydrobiologie 74, 423–451 (1987).
Wallace, J. B. et al. Effects of resource limitation on a detritial-based ecosystem. Ecological Monographs 69, 409–442 (1999).
Woodward, G. et al. Quantification and resolution of a complex, size-structered food web. Advances in Ecological Research 36, 85–135 (2005).