« Prev Next »

Introduction
Water current pervades every facet of existence for life in lotic (flowing water) habitats. Maintaining position in the face of flow can be energetically costly, but provides access to a conveyer belt-like food-delivery system. Stream and river organisms reflect their localized niche, and surrounding landscape both upstream and downstream. River organisms have evolved in diverse and fascinating ways in the varied environments between river source and mouth.Streams and Rivers: Habitats Partitioned at Different Spatial Scales
Large-scale differences: Source to mouth.
The blue line of a river on a map conveniently represents rivers as two-dimensional habitats beginning (usually) in a mountainous region, and ending in a far-off sea (or inland basin). But the physical changes in three dimensions along a river’s length have important implications for river inhabitants.
River sources are usually small, and in the case of mountain streams, steep and erosional (Montgomery and Buffington 1997). In temperate environments, small streams tend to be shaded by an interlocking, overhead tree canopy. Such conditions result in cool, well oxygenated streams that are abundantly supplied with a food base of leaves. Fine particles of organic matter are released as the leaves are broken down by biological communities in the streams (River Continuum Concept; Figure 1; Vannote et al. 1980).

At some point along their path to the sea, rivers have typically gained enough water and width to preclude interlocking tree canopies. This open-canopy state frequently coincides with somewhat lower gradient landscapes. Streams at this point are warmer, and less abundantly supplied with leaves than was the case upstream. These larger streams remain well oxygenated because air is entrained by turbulent flow in riffles. Open canopy, and fairly shallow water, means that light can reach the river benthos, increasing in-stream primary productivity.
Very large rivers are usually low gradient and very wide, resulting in negligible influence of riparian canopy in terms of shading and leaf-litter input. Water currents keep fine solids in suspension, reducing light penetration to the benthos. Organic matter in suspension is by far the largest food base in these very large rivers.
Changes in physical habitat and food base from river source to mouth profoundly influence biological communities. Aquatic ecologists classify benthic macroinvertebrates into functional feeding groups: shredders that eat leaves, collectors consuming fine particulates, grazers that scrape periphyton from substrates, and predators of animal prey (Cummins & Klug 1979). Smaller temperate streams tend to be co-dominated by shredders primarily consuming leaf litter, and collectors consuming particles (Figure 1). As canopies open in larger streams, grazers become common with increased periphyton production. With less canopy cover in wider streams, shredder abundance is reduced. Collectors utilize particles in streams of all sizes, but they dominate benthic communities in larger streams where suspended organic matter is common. Predators represent a small but important fraction of benthic communities in rivers of all sizes.
The fish zonation concept (Thienemann 1925, cited by Schmutz et al. 2000) generalized Western European river habitats based upon a predictable sequence of dominant fish species (Huet 1959). Analogous fish community responses to river slope and size have been found in African, South American, and many North American streams (McGarvey & Hughes 2008). Larger rivers can accommodate larger fish as well as small fish, and so the size range of fish increases as rivers become deeper. River discharge is the volume of water passing a particular location per unit time. The species-discharge relationship is analogous to the species-area relationship, and describes how fish diversity increases with river size (Figure 2; McGarvey & Milton 2008).
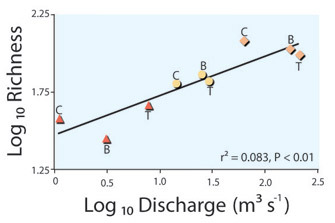
The river continuum and fish zonation concepts are idealized models of river systems that provide theoretical frameworks for hypothesis generation and comparisons to particular situations. When rivers pass through lakes, for example, water temperatures, the food base, and downstream communities are all modified (Ward & Stanford 1983). Many stream sources lie above the treeline, have reduced organic matter input, and differ from the predictions of the river continuum concept. Anthropogenic influences frequently increase particulate matter loading to streams, increasing filtering collector component of benthic communities.
Local scale structure: pools, riffles, meanders, and the hyporheic zone.
Local stream conditions vary substantially depending on gradient, stream size, and location along a stream continuum. A stream’s gradient profile can be generalized as gradually changing from steep to low gradient as we move from high to low elevation and from small to large rivers (Figure 3; Montgomery and Buffington 1997). Steep mountain streams cascade over large pieces of rocky substrate with almost constantly turbulent flow. When a steep stream is confined by valley walls, a series of pools separated by near-vertical steps can form. These step pools repeat at a frequency of approximately one to four channel widths.

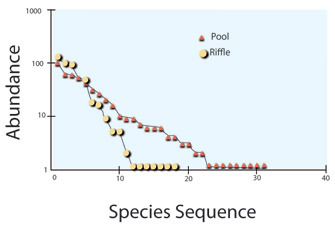
In low to moderate-gradient streams with loose rocky substrates, cobbles and boulders are mobilized during high-flow events and deposited across the width of river channels forming high-gradient riffles (Figure 4). Riffles are separated by pools, forming riffle-pool sequences recurring about three to five times the width of the river (Hynes 1970; Montgomery and Buffington 1997). During typical base-flow conditions, riffles are erosional habitats with fewer deposited fine particles between substrates. Particulate deposition increases as water velocity slows in pools. Riffle macroinvertebrate communities are typically more diverse than communities in pools. The pattern in fish communities is reversed, with pool fish communities tending to be more diverse than those in riffles (Figure 5; Gelwick 1990, Langeani et al. 2005).

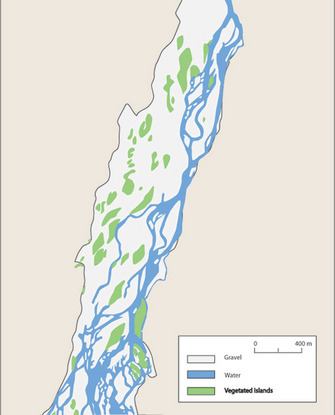
Larger alluvial rivers in their natural state are diverse habitats with side channels, sand and gravel bars, and islands that are formed and reformed on a regular basis (Figure 6; Ward & Tockner 2001). In low gradient flood plains, unconstrained rivers form meanders that shift and move as bed materials are eroded and redeposited. Dramatic changes can occur rapidly during flood events.
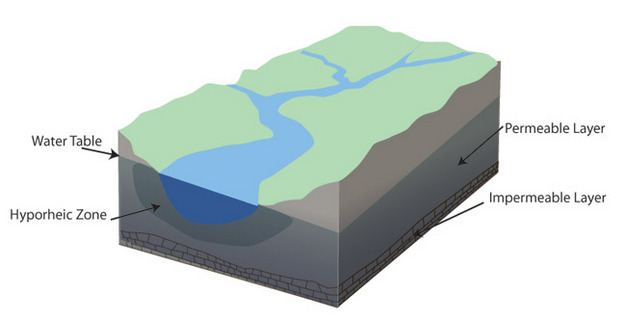
Streams exchange water, nutrients, and organisms with surrounding aquifers. The interstitial, water-filled space beneath river beds, where most active aquifer-river water exchange occurs, is termed the hyporheic zone, and is an important habitat for a number of aquatic organisms (Figure 7; Gibert et al. 1994, and references therein). The limits of the hyporheic zone vary, and riverine organisms can be found in groundwater up to 2 km from active stream channels (Stanford & Ward 1988).
Gradients in physical characteristics, including flow, depth, substrate characteristics, and light penetration, exist across river channels. These physical differences within a river result in a diverse range of potential niches for aquatic organisms. Because of the heterogeneous nature of riverbeds, the distributions of fish, invertebrates, and algae, tend to form a patchy mosaic that shifts and responds dynamically to high-water events (Townsend 1989). One result of this patchiness is that samples of river organisms are notoriously variable.
Small scale differences: Microhabitats.
Organisms distribute themselves at even smaller spatial scales than those described above. The size and texture of river substrates influence invertebrate abundance and species richness (Downes et al. 1995). Invertebrate communities respond to different combinations of velocity, depth, and substrate roughness (Brooks et al. 2005). As is true in other habitats, the distributions of river organisms are additionally influenced by biological interactions.
Exchange With Surrounding Environments
Rivers exchange water, materials, energy, and nutrients, in a reciprocal manner with the surrounding environment. River water quality, sediment characteristics, and biological communities, all reflect characteristics of the upstream, and even the downstream environment. Conversely, local environments are thermally influenced, sculpted, watered, and nutritionally supplemented, by rivers and streams.
Soil deposition by rivers onto their floodplains has influenced the course of human agriculture, and the distributions of human populations since antiquity. River influence is strongest on the immediately-adjacent habitat called the riparian zone. Biological communities in riparian zones are unique, and distinct from those beyond the immediate influence of rivers. In some biomes such as deserts and grasslands, river corridors are the most vegetated habitats that exist, and provide essential habitat for a range of organisms. Riparian woods serve as important wildlife migration corridors linking fragmented forests (Lees and Peres 2008).
Upstream migrations of anadromous fish species bring marine-derived nutrients to the lotic environment (Wold & Hershey 1999). Fish carcasses increased insect abundance eight-fold in one experiment (Wipfli et al. 1998). Aquatic insects in turn transfer nutrients linking food webs between rivers and their riparian zones (Nakano & Murakami 2001). Larval aquatic insects spend weeks, more typically a year, or even longer, in streams before adult emergence. The synchronous insect emergences sought by trout anglers, and indeed by trout, are sometimes large enough to be detected by regional weather radar (Figure 8), and provide vital nutrition for fish, terrestrial invertebrates, birds, and mammals. Terrestrial insects falling into streams constitute important parts of the diets of stream fish, making up as much as 50% of the diet of Dolly Varden Char (Salvelinus malma) in one study (Nakano et al. 1999).

Tree limbs that fall into streams and rivers increase habitat heterogeneity. Submerged woody debris persists for long periods in streams and rivers, with a calculated half-life of ~ 20 years (Hyatt & Naiman 2001). Woody debris can stabilize river beds, modify erosion and deposition, create essential fish habitat, and help form pools that retain organic matter, extending the availability of seasonal food resources. Experimentally manipulated woody debris was shown to increased both macroinvertebrate and fish colonization (Angermeier & Karr 1984).
Stormwater runoff from surrounding landscapes carries particles into streams. The particles include soil as well as plant and animal detritus. Organic particles in the runoff contribute to the food base in stream and river ecosystems.
Disturbance and Community Interactions
Heavy rainfall and snowmelt can greatly magnify the volume of stream water in a relatively short period of time. Rapidly flowing water can carry large quantities of sand and gravel, effectively sand-blasting surfaces, and removing the periphyton layer. It is not unusual to see macroinvertebrate abundances reduced by half, or more, following such high-water events.
Reviewers of the river literature have generally concluded that disturbance is of greater importance than species interactions in streams (Lake 1990, Resh et al. 1988). This conclusion does not imply that community interactions are unimportant, and well-studied examples abound in the scientific literature, but the impacts of disturbance are generally considered to be of greater magnitude. Importantly, river organisms have evolved with a context of natural disturbance, and communities persist in spite of it.
Human Influences
Humans have rapidly introduced a wide array of disturbances to which river organisms have had no previous exposure during their evolution. We have dammed, channelized, diverted, drained, filled, and polluted, streams and rivers. We have removed riparian vegetation, paved extensive portions of river catchments, and isolated river systems from their floodplains. Water is transferred among river basins, and river organisms are exchanged among continents. Our influence on rivers has been so pervasive that one research group (Vörösmarty et al. 2010) estimated that fully 65% of the river water discharging to our oceans is associated with threatened habitats.
Our influences on river systems alter the nature of rivers and affect all of the processes described above. It is important to note that, because river systems are well studied, environmental engineers have a sound, scientific basis for designing river restoration projects. The general frameworks described above, along with others beyond the scope of this article, provide scientific benchmarks against which to measure the success of restoration efforts.
Summary
The science of life in flowing water is well developed and active. River research is multidisciplinary in nature, and draws from many branches of biology, chemistry, physics, and engineering. Because the vast majority of streams and rivers are in some way managed by human populations, our impacts on these systems can be extensive, as is our potential to improve stream habitat quality. Career opportunities for the next generation of river scientists exist in a wide variety of fields, and these new river scientists will have important influences on the health of rivers in the years to come.
Glossary
Alluvial: Referring to loose inorganic substrates such as sand, gravel, and boulders eroded, transported, and deposited and often sorted by the action of water.
Anadromous: Fish spending most of their life cycle in salt water and migrating to freshwater to reproduce.
Aquifer: Underground water that exists in the interstitial space between substrate particles or porous rock.
Benthos: The community of organisms inhabiting the solid floor, or benthic zone of any water body.
Biomes: Large biogeographical regions characterized by a particular community type. They are broadly defined by climatic variables including temperature and precipitation. Examples include desert, rain forest, and tundra.
Catchment: The area that drains to a single stream or river. Frequently referred to as a river basin. Synonymous with watershed in North American usage.
Collectors: A macroinvertebrate functional feeding group using small organic particles as a primary food source. Filtering collectors accumulate this material from the water column. Gathering collectors accumulate this material from the benthic zone.
Discharge: The quantity of water passing a certain river or stream location per unit time. Expressed as units of volume per unit time (e.g. cubic meters per second).
Feeding guilds: Organisms categorized by their feeding mode. Examples include nectar feeders, and parasites. See functional feeding groups.
Functional feeding groups: Feeding guilds of aquatic macroinvertebrates. These include grazers (commonly called scrapers), shredders, collectors, and predators.
Grazers: Also called scrapers, a macroinvertebrate functional feeding group that consumes attached periphyton as its primary food source
Hyporheic zone: A zone of saturated substrate beneath and spreading laterally from a river bed. It is the zone of active water and organism exchange between the river water and ground water.
Lentic: Referring to standing-water habitats including lakes, ponds, and swamps (contrast with lotic).
Lotic: Referring to flowing-water habitats including rivers, springs, and streams (contrast with lentic).
Periphyton: The community of primary producers and heterotrophic microorganisms attached to submerged surfaces. In streams this would include algae, cyanobacteria, bacteria, and fungi and their associated extra-cellular secretions. Periphyton serves as the food base utilized by grazers.
Pool: An area of low gradient water in a stream. See also riffle.
Predators: Organisms whose primary food source is other animals.
Riffle: A high-gradient bar of deposited substrate, usually spanning the width of a stream. Typically found as part of a riffle-pool repeating sequence in streams of medium gradient. Not to be confused with ripple.
Ripple: Small-scale undulations on the surface unconsolidated fine substrates such as silt and sand. These features are shaped by the action of flowing water in low-gradient rivers.
Riparian zone: The area of terrestrial habitat adjacent to and most directly influenced by a river or stream.
River Continuum Concept: A model of longitudinal change in physical habitat, and the biological communities in rivers.
Shredders: A benthic macroinvertebrate functional feeding group that utilizes leafy detritus as their primary food source. Although the leaves are consumed, nutritional value is derived from the attached community as well as the leaves themselves.
Species-area relationship: The frequently-confirmed observation that as one increases the area from which a community sample is taken, one typically samples an increasing number of species.
Species-discharge relationship: An analogous concept to the species-area concept applied to rivers. As river size (as measured by discharge) increases, one usually finds more fish species.
Step pools: A series of stream pools separated by areas of high-gradient water flow. These pools can be visualized by analogy to the step surfaces on stairs separated by the stair risers that support those steps.
Watershed: European usage: the boundary between two catchments; water is shed to one stream on one side of this line and in the opposite direction and to a different stream on the other side. North American usage: a synonym of catchment.
References and Recommended Reading
Brooks, J. A. et al. Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles. Freshwater Biology 50, 331-344 (2005).
Cummins, K. W. & Klug, M. J. Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics 10, 147-172 (1979).
Downes, B. J. et al. Habitat structure and invertebrate assemblages on stream stones: A multivariate view from the riffle. Australian Journal of Ecology 20, 503-514 (1995).
Frissell, C. A. et al. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environmental Management 10, 199-214 (1986).
Gelwick, F. P. Longitudinal and temporal comparisons of riffle and pool fish assemblages in a Northeastern Oklahoma Ozark stream. Copeia 1990, 1072-1082 (1990).
Gibert, J. et al. eds. Groundwater Ecology: Aquatic Biology. San Diego, CA: Academic Press, 1994.
Huet, M. Profiles and biology of Western European streams as related to fish management. Transactions of the American Fisheries Society 88, 155-163 (1959).
Hyatt, T. L. & Naiman, R. J. The residence time of large woody debris in the Queets River, Washington, USA. Ecological Applications 11, 191-202 (2001).
Hynes, H. B. N. The Ecology of Running Waters. Toronto, Canada: University of Toronto Press, 1970.
Lake, P. S. Disturbing hard and soft bottom communities: A comparison of marine and freshwater environments. Australian Journal of Ecology 15, 477-488 (1990).
Langeani, F. et al. Riffle and pool fish communities in a large stream of southeastern Brazil. Neotropical Ichthyology 3, 305-311 (2005).
Lees, A. C. & Peres, C. A. Conservation value of remnant riparian forest corridors of varying quality for amazonian birds and mammals. Conservation Biology 22, 439-449 (2008)
McGarvey, D. J. & Hughes, R. M. Longitudinal zonation of Pacific Northwest (U.S.A.) fish assemblages and the species-discharge relationship. Copeia 2008, 311-321 (2008).
McGarvey, D. J. & Ward, G. M. Scale dependence in the species-discharge relationship for fishes of the southeastern U.S.A. Freshwater Biology 53, 2206-2219 (2008).
Montgomery, D. R. & Buffington, J. M. Channel-reach morphology in mountain drainage basins. Geological Society of America Bulletin 109, 596-611 (1997).
Nakano, S. et al. Terrestrial-aquatic linkages: Riparian arthropod inputs alter trophic cascades in a stream food web. Ecology 80, 2435-2441 (1999).
Nakano, S. & Murakami, M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences of the United States of America 98, 166-170 (2001).
Resh, V. H. et al. The role of disturbance in stream ecology. Journal of the North American Benthological Society 7, 433-455 (1988).
Schmutz, S. et al. A multi-level concept for fish-based, river-type-specific assessment of ecological integrity. Hydrobiologia 422, 279-289 (2000).
Stanford, J. A. & Ward, J. V. The hyporheic habitat of river ecosystems. Nature 335, 64-66 (1988).
Thienemann, A. Die Binnengewässer Mitteleuropas eine Limnologische Einführung. Stuttgart, 1925.
Townsend, C. R. The patch dynamics concept of stream community ecology. Journal of the North American Benthological Society 8, 36-50 (1989).
Vannote, R. L. et al. The river continuum concept. Canadian Journal of Fisheries and Aquatic Science 37, 130-137 (1989).
Vörösmarty, C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555-561 (2010).
Ward, J. V. & Stanford, J. A. "The serial discontinuity concept of lotic ecosystems," in Dynamics of Lotic Ecosystems, eds. T. D. Fontain and S. M. Bartell (Ann Arbor, MI: Ann Arbor Science Group, 1983) 29-42.
Ward, J. V. & Tockner, K. Biodiversity: Towards a unifying theme for river ecology. Freshwater Biology 46, 807-819 (2001).
Wold, A. K. F. & Hershey, A. E. Effects of salmon carcass decomposition on biofilm growth and wood decomposition. Canadian Journal of Fisheries and Aquatic Sciences 56, 767-773 (1999).






























