« Prev Next »

Reconstructing the evolution of human development from a severely limited fossil record may be viewed as both a fundamental challenge and a promising field of research. Life history, or the way in which organisms time and allocate energy to growth, reproduction, and maintenance, is fundamental to a species' biology and behavior. Human life history differs in many ways from our closest living relatives, the African great apes. In particular, we have a longer gestation, earlier weaning, slower postnatal maturation, older age at first reproduction, and longer post-reproductive period (Bogin 1990). This developmental pattern also impacts demography, or population structure, and may help us to better understand how natural selection facilitated our species' remarkable success, while our closest living relatives are largely on the brink of extinction.
It might seem that life history is invisible in the fossil record, but many primate life history variables correlate strongly with the development of the brain and dentition (Smith 1989, Godfrey et al. 2001, Kelley & Smith 2003, Dirks & Bowman 2007, DeSilva & Lesnik 2008, Kelley & Schwartz, in press). Life history variables are often inferred for fossil species from related variables such as body and brain mass, as well as tooth eruption ages. For example, the age at which the first molar erupts into the mouth closely predicts age at weaning across primate species, whereas third molar eruption age is highly correlated with age at first reproduction (Smith 1989, Smith et al. 1994, Kelley & Smith 2003). Thus, knowledge of these eruption ages may allow scientists to model the rate of population increase, for example. Furthermore adult brain size is correlated with age at reproductive maturation, although it is unclear whether the brain is a direct pacesetter of life histories (Leigh & Blomquist 2007, Robson & Wood 2008).
Until recently, extant reference taxa such as humans and apes have been used to model the evolution of human development, presuming that extinct hominins followed similar developmental trajectories (Conroy & Vannier 1991, Smith & Tompkins 1995). Recent studies of fossil taxa that do not appear to fit either group, such as Homo erectus (DeSilva & Lesnik 2008, Dean & Smith 2009), underscore the fact that the use of extant models is not ideal. Fossil hominins evolved in a remarkable range of environments and possessed unique anatomical specializations, and it is likely that growth patterns in certain taxa were unlike those of primate species living today. While the correlations detailed above explain variation across a wide sample of primates, recent studies have begun to critically explore how well brain size and tooth development explain life history variation within living apes and humans (Robson & Wood 2008, Guatelli-Steinberg 2009,Kelley & Schwartz, in press). Moreover, while it is well established that humans are developmentally unique among living primates, weaning earlier and reproducing later than expected, the adaptive significance of our prolonged childhood remains unresolved (Smith & Tompkins 1995). Below we detail how recent developments in computed tomography (CT) and synchrotron imaging are helping scientists tackle some of these challenges via age determination in juvenile fossils, yielding assessments of brain growth and tooth development that are independent of living reference populations. Ultimately, this research aims to provide insight into the evolutionary relationships of fossil hominins, as well as the selective mechanisms, adaptive responses, and environmental context that led to the evolution of Homo sapiens.
Brain Growth and Mass
Humans are unique among primates in their large adult brain size relative to body size (encephalization quotient). When compared to the entire hominin lineage, currently dated to as far back as 6-7 million years, exceptionally large brains are only first observed around 300,000 years ago. What triggered this expansion and how it relates to changes in life history are still debated. Because soft-tissue impressions are rarely preserved, scientists often measure the space within the braincase as a proxy for brain size, which is known as the endocranial volume (EV). Proportional EV, representing the ratio between an immature volume and an average adult volume, reveals the relative amount of brain formed at a given developmental stage. Both absolute and proportional EVs are used to examine patterns of brain development and their relation to other behavioral and somatic developmental patterns. A proportionately small brain at birth in a species with a very large adult brain typically requires more investment from the mother and/or other caregivers. Large adult brain sizes are achieved by high rates and/or long durations of brain development. These pathways are variable in primates and are not fully explained by evolutionary relationships (Leigh 2004).
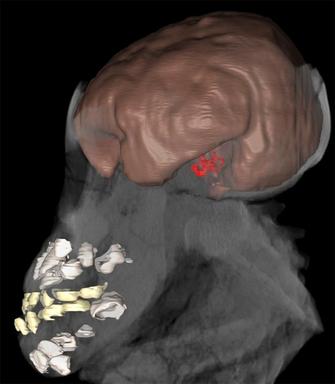
While data on adult EVs are available for many fossil hominin species, the lack of information on neonatal and juvenile EV is the main obstacle to studying patterns of brain growth evolution (Leutenegger 1987, Hausler & Schmid 1995, DeSilva & Lesnik 2008). Recent work has examined brain development in a small sample of juvenile hominins, including Australopithecus afarensis, Homo erectus, and Homo neanderthalensis, which may have implications for the evolution of human cognition (Coqueugniot et al. 2004, Alemseged et al. 2006, Coqueugniot & Hublin 2007, Ponce de Leon et al. 2008, Gunz et al. 2010). For example, Alemseged et al. (2006) suggested that brain growth in Australopithecus afarensis might have been slightly different from that observed in apes, although absolute adult brain sizes do not differ considerably. This was based on the DIK-1-1 juvenile, with a preliminary estimated age at death of 3 yr and EV of 275-330 cubic centimeters (cc) (Figure 1). Both DIK-1-1 and A.L. 333-105 (another juvenile from this species) fall below the average proportional EV of similarly aged African apes, and are more similar to modern humans. Alemseged et al. (2006) interpreted this as evidence of slower absolute brain growth that would have continued over a slightly longer period than in apes. Ongoing work detailed below will yield more accurate estimates of EV and age at death in DIK-1-1 that are independent of ape or human models, which is critical for determining if this species may have differed from the primitive hominin condition.
DeSilva & Lesnik (2008) recently showed that brain masses are good predictors of life history variables across taxa. By employing the scaling relationship between neonatal and adult brain size demonstrated by Martin (1983, 1990), they predicted brain size at birth for australopiths, early Homo, and Homo erectus (180, 225, and 270 cc, respectively). Proportional EVs derived from these values suggest that modeling early hominin brain development patterns after that of either living humans or chimpanzees is not warranted, as there seems to be a gradual change, with later hominins becoming more human-like. While considerable methodological debate exists (e.g., Hublin & Coqueugniot 2006, Leigh 2006, Leigh & Blomquist 2007, Robson & Wood 2008), none of the fossil species studied to date appear to match the modern human trajectory of brain growth (rate and/or duration), which may represent another unique character that aided our evolutionary success.
Dental Microstructure
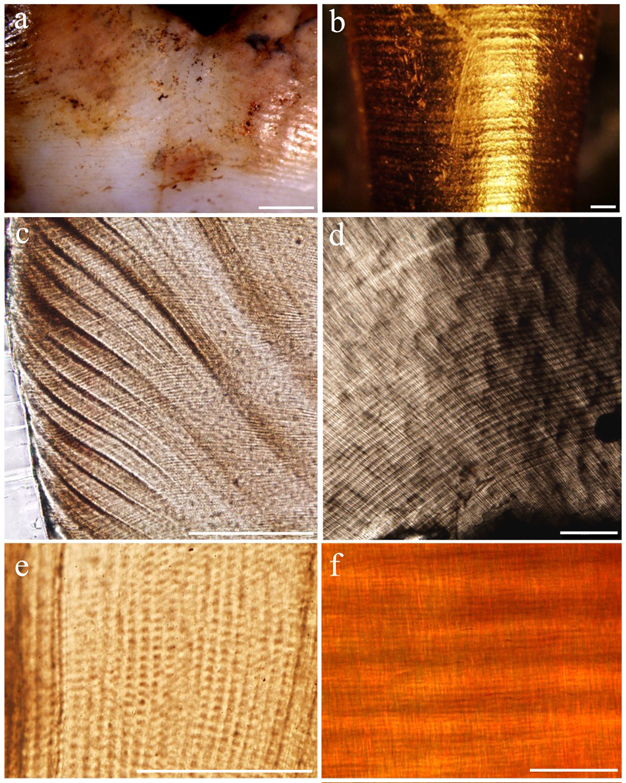
Owing to their highly mineralized nature, teeth are the best preserved elements in fossil assemblages. Valuable information is recorded on tooth crowns and roots, providing a permanent temporal record similar to the rings in tree trunks. Tiny lines are laid down during enamel and dentine secretion, which faithfully record the speed of growth every day as these hard tissues take shape (Figure 2). Humans and great apes begin growing their teeth prior to birth, continue this process throughout adolescence, and complete it by the beginning of adulthood. Records of rhythmic growth and developmental stress, including a line formed during birth (neonatal line), remain unchanged for millions of years. Tooth histology, or quantification of microscopic growth, is the most effective means of determining eruption ages and age at death in juvenile hominins (Dean 2006, Smith 2008). These data may provide insight into population-level developmental variation, mortality rates, demographic structure, and living conditions of prehistoric cultures. Importantly, the pace of tooth growth tracks the timing of various life history variables (Smith 1989, Godfrey et al. 2001; Kelley & Smith 2003, Dirks & Bowman 2007, Kelley & Schwartz, in press). Without histological age determination, independent benchmarks for cranial and postcranial maturation remain elusive in fossil species, as there are no alternative methods to age juveniles without employing living reference populations.

Glossary
Andresen lines: Long-period (greater than circadian) incremental features in dentine representing the successive positions of the dentine-forming front, which correspond to periradicular bands on the root surface, and are temporally equivalent to Retzius lines in enamel. (See example in Figure 2.)
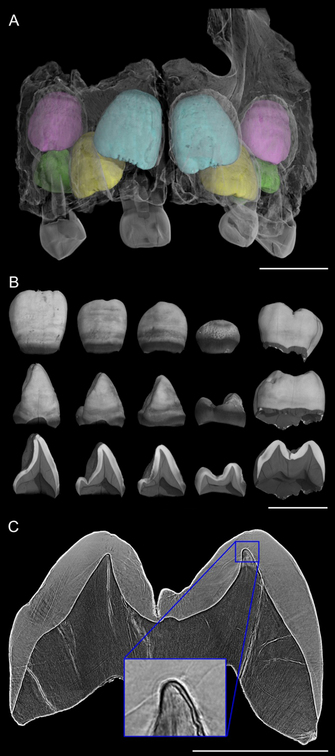
computed tomography (CT): Imaging method that uses an X-ray source to create a series of radiographic projections captured over a series of angles (due to rotation of the sample or the source and detector), which are then reconstructed with a computer program to yield virtual cross-sectional slices through an object. Conventional laboratory or medical CT relies on differential X-ray absorption to yield slices with pixel values that correspond to density differences in the sample. These slices may be used to create a three-dimensional model of the object that was scanned. (See example in Figure 1.)
cross-striations: Short-period (i.e., circadian or shorter) incremental features in enamel prisms. Cross-striations represent a 24-h cycle of cellular activity, and thus the distance between adjacent cross-striations yields the enamel daily secretion rate. (See example in Figure 2.)
deciduous dentition: The primary, or first, set of teeth that begins forming prior to birth and is replaced with the permanent dentition during childhood. Humans and apes have a total of 20 deciduous teeth. (See example in Figure 1.)
dental development: The continuous process of tooth initiation, matrix secretion, crown calcification, root completion, and dental eruption. Primate dental development begins prior to birth with the initiation of the deciduous dentition, followed by replacement with the permanent dentition during childhood.
dentine: Mineralized tissue that makes up the inner core of a tooth crown, as well as the tooth root, which helps to anchor teeth into jaws. Dentine is formed when cells (odontoblasts) secrete a collagenous matrix that undergoes mineralization. Dentine formation begins at the dentine horn, underlying the future cusp tip, and progresses inward through secretion and downward through extension until it reaches the apex of the root. Fine microscopic incremental features such as von Ebner's lines and Andresen lines mark the temporal progress of dentine secretion.
enamel: Highly mineralized tissue that makes up the outer coating of a tooth crown, giving teeth their characteristic shapes. Enamel is formed when cells (ameloblasts) secrete enamel matrix proteins that mineralize into long thin bundles of crystallites known as enamel prisms. As the cells progress outward towards the future tooth surface, additional cells are activated through extension until the forming front reaches the cervix of the crown. Fine microscopic incremental features such as cross-striations and Retzius lines mark the temporal progress of enamel secretion.
gestation: The carrying of an embryo or fetus during pregnancy. The period of gestation is the time between conception and birth.
neonatal line: A pronounced line corresponding to the position of the enamel and dentine fronts in teeth developing during birth, caused by the profound physiological changes occurring then. This line in found in the entire deciduous dentition, as well as the permanent first molar of apes and humans. (See example in Figure 4.)
perikymata: External ridges and troughs encircling the tooth crown that are formed by Retzius lines as they reach the enamel surface. (See example in Figure 2.)
periradicular bands: External ridges and troughs encircling the tooth root that are formed by Andresen lines as they reach the root surface. (See example in Figure 2.)
permanent dentition: The second set of teeth that replace the deciduous dentition during childhood. Of these, only the first molar begins forming prior to birth, and thus contains a neonatal line that may be used to age a developing dentition. Humans and apes have a total of 32 permanent teeth. (See example in Figure 4.)
Retzius lines: Long-period incremental features in enamel that represent the position of the developing enamel front at successive points in time. They manifest on the tooth surface as perikymata, and are analogous to the Andresen lines in dentine. The temporal repeat interval of Retzius lines is known as their periodicity, the number of days between pairs of lines. Knowledge of this rhythm is necessary for accurate reconstruction of crown formation time. (See example in Figure 2.)
synchrotron: Machine that produces beams of light from accelerated electrons deviated by magnetic fields. Depending on the energy, the light spectrum may range from radio frequencies to high-energy X-rays. This type of source facilitates more efficient and diverse imaging possibilities than conventional laboratory X-ray sources. A unique and critical aspect of synchrotron imaging for dental tissue research is based on phase-contrast techniques, which reveal subtle variations in tissue structure that are invisible with other absorption contrast imaging techniques such as radiography or conventional computed tomography.
tooth eruption: The process of emergence where the tooth crown moves past the bone margin (alveolar eruption) and the gum (gingival eruption) in order to emerge into the oral cavity, and eventually into functional occlusion. The age at which first and third molars erupt into the oral cavity shows a high correlation with certain life history characteristics across primates (Smith 1989).
von Ebner's lines: Short-period incremental features in dentine that reflect a 24-h cycle of dentine secretion, and are temporally equivalent to cross-striations in enamel. (See example in Figure 2.)
weaning: The process characterizing the cessation of nursing, or provisioning of milk via lactation. Following a period of exclusive mother's milk consumption, nursing duration and frequency may fluctuate as juveniles transition to a solid food diet, thus weaning is sometimes described as a process rather than an event.
References and Recommended Reading
Alemseged, Z. et al. A juvenile early hominin skeleton from Dikika, Ethiopia. Nature 443, 296-301 (2006).
Bogin, B. The evolution of human childhood. BioScience 40, 1-25 (1990).
Bogin, B., & Smith, B.H. Evolution of the human life cycle. American Journal of Human Biology 8, 703-716 (1996).
Bromage, T. G., & Dean, M. C. Re-evaluation of the age at death of immature fossil hominids. Nature 317, 525-527 (1985).
Coqueugniot, H. et al. Early brain growth in Homo erectus and implications for cognitive ability. Nature 431, 299-302 (2004).
Coqueugniot, H. & Hublin, J. J. Endocranial volume and brain growth in immature Neandertals. Periodic Biology 109, 379-385 (2007).
Conroy, G. & Vannier, M. W. Dental development in South African australopithecines. Part II: Dental stage assessment. American Journal of Physical Anthropololgy 86, 137-156 (1991).
Dean, M. C. Tooth microstructure tracks the pace of human life-history evolution. Proceedings of the Royal Society B 273, 2799-2808 (2006).
Dean, C. et al. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature 414, 628-631 (2001).
Dean M. C. & Smith, B. H. "Growth and development in the Nariokotome youth, KNM-WT 15000," in The First Humans: Origin of the Genus Homo, eds Grine, F. E., Fleagle, J. G. & Leakey, R. E (New York, NY: Springer, 2009) 101-120.
DeSilva, J. M. & Lesnik, J. J. Brain size at birth throughout human evolution: a new method for estimating neonatal brain size in hominins. Journal of Human Evolution 55, 1064-1074 (2008).
Dirks, W. & Bowman, J. E. Life history theory and dental development in four species of catarrhine primates. Journal of Human Evolution 53, 309-320 (2007).
Godfrey, L. R. et al. Teeth, brains, and primate life histories. American Journal of Physical Anthropology 114, 192-214 (2001).
Guatelli-Steinberg, D. Recent studies of dental development in Neandertals: Implications for Neandertal life histories. Evolutionary Anthropology 18, 9-20 (2009).
Gunz, P. et al. Brain development after birth differs between Neanderthals and modern humans. Current Biology 20, R921-R922 (2010).
Harvey, P.H. & Clutton-Brock, T.H. Life history variation in primates. Evolution 39, 559-581 (1985).
Hausler, M. & Schmid, P. Comparison of the pelves of Sts 14 and AL 288-1: Implications for birth and sexual dimorphism in australopithecines. Journal of Human Evolution 29, 363-383 (1995).
Hawkes, K. & Paine, M. (eds.) The Evolution of Human Life History. Santa Fe, NM: School of American Research Press, 2006.
Hublin J. J. & Coqueugniot, H. Absolute or proportional brain size: that is the question. A reply to Leigh's (2006) comments. Journal of Human Evolution 50, 1-5 (2006).
Kaplan, H. et al. A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology 9, 156-185 (2000).
Kelley, J. & Schwartz, G.T. Life-history inference in the early hominins
Australopithecus and Paranthropus. International Journal of Primatology (in press).
Kelley, J. & Smith, T. M. Age at first molar emergence in early Miocene Afropithecus turkanensis and life-history evolution in the Hominoidea. Journal of Human Evolution 44, 307-329 (2003).
Leigh, S.R. Brain growth, life history, and cognition in primate and human evolution. American Journal of Primatology 62, 139-164 (2004).
Leigh, S. R. Brain ontogeny and life history in Homo erectus. Journal of Human Evolution 50, 104-108 (2006).
Leigh, S. R. & Blomquist, G. E. "Life history," in Primates in Perspective, eds. Campbell C. J. et al. (Oxford, UK: Oxford University Press, 2007) 396-407.
Leutenegger, W. Neonatal brain size and neurocranial dimensions in Pliocene hominids: Implications for obstetrics. Journal of Human Evolution 16, 291-296 (1987).
Martin, R. D. Human Brain Evolution in an Ecological Context: Fifty-Second James Arthur Lecture on the Evolution of the Human Brain. New York, NY: American Museum of Natural History, 1983.
Martin, R. D. Primate Origins and Evolution. Princeton, NJ: Princeton University Press, 1990.
Martin, R. D. Scaling of the mammalian brain: The maternal energy hypothesis. News in Physiological Sciences 11, 149-156 (1996).
Ponce de Leon, M. S. et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proceedings of National Academy of Sciences (USA) 105, 13764-13768 (2008).
Robson, S. L. & Wood, B. Hominin life history: Reconstruction and evolution. Journal of Anatomy 212, 394-425 (2008).
Smith, B. H. Dental development as a measure of life history in primates. Evolution 43, 683-688 (1989).
Smith, B.H. et al. Ages of eruption of primate teeth: a compendium for aging individuals or comparing life histories. Yearbook of Physical Anthropology 37,177-231 (1994).
Smith, B. H. & Tompkins, R. L. Toward a life history of the Hominidae. Annual Review of Anthropology 24, 257-279 (1995).
Smith, T. M. Incremental dental development: methods and applications in hominoid evolutionary studies. Journal of Human Evolution 54, 205-224 (2008).
Smith, T. M. et al. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proceedings of the National Academy of Sciences (USA) 107:20923-20928 (2010).
Smith, T. M. et al. Rapid dental development in a Middle Paleolithic Belgian Neanderthal. Proceedings of the National Academy of Sciences (USA) 104, 20220-20225 (2007).
Stringer, C. B. et al. "A comparative study of cranial and dental development within a recent British sample and among Neandertals," in Primate Life History and Evolution, ed. C. J. de Rousseau (New York: Wiley-Liss, 1990) 115-152.
Tafforeau, P. et al. Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Applied Physics 83, 195-202 (2006).
Tafforeau, P. & Smith, T. M. Nondestructive imaging of hominoid dental microstructure using phase contrast X-ray synchrotron microtomography. Journal of Human Evolution 54, 272-278 (2008).