« Prev Next »

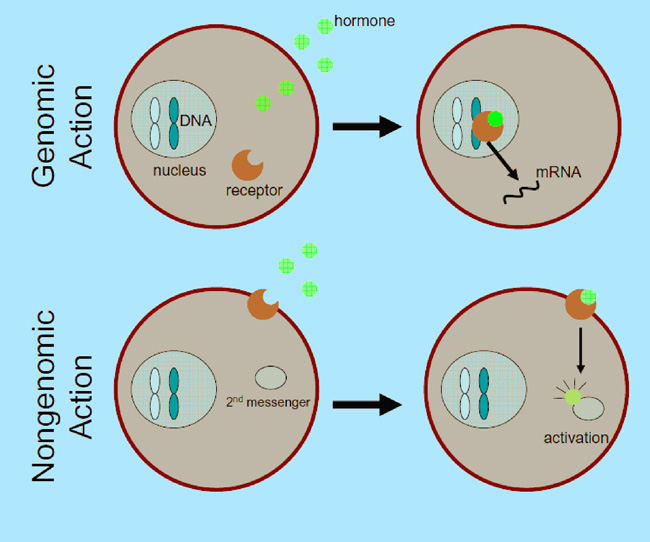
A widely used method for determining whether steroid hormones, like testosterone, affect aggression is to conduct long-term manipulations. Manipulations, such as castration and/or applying hormone implants, are effective approaches to determining whether steroid hormones regulate behavior. When students are taught about the mechanisms of steroid hormones, they usually learn about how steroid hormone receptors act as transcription factors, regulating the expression of genes within the nucleus of a cell (Figure 1). This is a process that occurs over hours or days, and the scientific community's focus on these mechanisms began in the 1960s with the discovery of steroid receptors in the nucleus (Pfaff & Levine 2008). This change in focus shifted attention away from more rapid mechanisms of steroid action for several decades. Rapid mechanisms can occur within seconds or minutes of a change in steroid concentrations, and are generally considered to be independent of receptors acting in the nucleus (Figure 1). However, there has been a revival of interest in rapid actions of steroids on behavior (Vasudevan & Pfaff 2006). As outlined below, recent discoveries have shown that steroid hormones can influence a wide variety of behaviors within minutes, presumably through these so-called nongenomic mechanisms. Detecting rapid effects of steroid hormones requires different approaches to complement the widely used experiments using castration and home implant manipulations.
Stress
A seminal study in Frank Moore's lab showed that acute exposure to either endogenous or exogenous corticosterone exposure caused male rough-skinned newts (Taricha granulosa) to exhibit reduced clasping of females, a courtship behavior that typically lasts hours or days (Moore & Miller 1984). Importantly, the inhibition of this behavior occurs eight minutes after corticosterone exposure (Orchinik et al. 1991). Membrane-bound corticosterone receptors, which do not migrate to the nucleus, were also discovered in the newt brain (Orchinik et al. 1991, 1992). These findings are important because they show that reproductive behavior can be rapidly downregulated following the release of corticosterone. Many mating and courtship behaviors are conspicuous, which increases the risk of predation (Cooper 1999). This may explain why stress-response systems that are activated by predators quickly downregulate reproductive activities in the face of threats. In female sheep, cortisol rapidly renders the pituitary insensitive to gonadotropin releasing hormone released by the hypothalamus (Breen & Karsch 2004). This is important because hormones released by the pituitary facilitate female mating behaviors (Blaustein 2010).

Courtship
In zebra finches, estradiol is primarily synthesized in the brain. However, steroids from the gonads can also exert rapid effects on behavior. Male goldfish (Carassius auratus) show a rapid rise in plasma testosterone (an androgen hormone) upon encountering ovulating females (Kobayashi et al. 1986). This increase in testosterone is important because other studies demonstrate that an injection of testosterone increases the frequency that males approach females (Lord et al. 2009). Injections of estradiol had similar effects on male behavior. This observation is important because the aromatase enzyme converts testosterone into estradiol. This study also showed that an aromatase inhibitor, which blocks the conversion of testosterone into estradiol, prevented the positive effect of testosterone on male approach behavior. (Lord et al. 2009). Together these data indicated that testosterone released by the testes is converted into estradiol within the brain, which then rapidly alters behavior. Circulating androgens also act rapidly to increase male advertisement vocalizations in the Gulf toadfish (Remage-Healey & Bass 2006). Thus, it appears that androgens released in the presence of females can act rapidly to modulate male courtship behavior, which could presumably have important effects on reproductive success.
Aggression
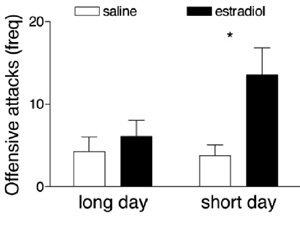
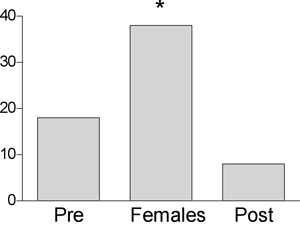
Field studies on song sparrows (Figure 6) have demonstrated that estrogens increase male aggression in the winter (Soma et al. 2000b), which is consistent with the laboratory data from Peromyscus. Furthermore, inhibition of estradiol synthesis reduced aggression within 24 hours (Soma et al. 2000a), which is a relatively fast effect for field studies. In the winter, male song sparrow gonads atrophy and testosterone levels are extremely low. However, in the winter the adrenal gland produces elevated levels of dehydroepiandrosterone (DHEA)(Soma & Wingfield 2001), which is an androgen precursor. The brain can convert DHEA to androgens, and this process is specifically enhanced during aggressive challenges (Pradhan et al. 2010). Interestingly, DHEA is elevated during the winter in territorial red squirrels (Boonstra et al. 2008) and aggressive encounters increase DHEA levels in hamsters (Scotti et al. 2009). These data suggest that DHEA could be an important hormone regulating aggression in a wide array of species. It has been hypothesized that androgen synthesis in the brain allows individuals to engage in territorial behaviors that are mediated by androgens (and their estrogenic metabolites) while avoiding the metabolic costs of high testosterone levels.
Conclusion
Steroid hormones can modify behavior through protein synthesis pathways, which is a potent and enduring approach to responding to a changing environment. However, as we have shown, there are circumstances wherein more rapid, short-term responses are needed. Recent experiments have demonstrated that steroid hormones can indeed exert rapid effects on behavior, although the mechanisms for these effects differ from pathways involving long-term changes in gene expression. This could be beneficial, because some steroids, such as testosterone, are known to exert detrimental side effects. For example, long-term increases in plasma testosterone increase energy expenditure (Marler et al. 1995) and may suppress the immune system (Mills et al. 2010). Rapid nongenomic pathways permit steroid hormones to regulate behavior in response to sudden and short-lived environmental or social change. In this manner, a sudden encounter with a predator can prevent a rough-skinned newt from initiating a protracted mating ritual that leaves it vulnerable, as a corticosterone rapidly alters brain activity to suppress mating behavior As the literature builds, it is becoming clearer that steroid hormones are important in both short- and long-term regulation of behaviors.
References and Recommended Reading
Beach, F. A. & Inman, N. G. Effects of castration and androgen replacement on mating in male quail. Proceedings of the National Academy of Sciences USA 54, 1426–1431 (1965).
Blaustein, J. D. "Feminine reproductive behavior and physiology in rodents: integration of hormonal, behavioral, and environmental influences." In Hormones Brain and Behavior, 2nd ed., eds. Donald W. Pfaff et al. (New York, NY: Academic Press): 67–107.
Bonier, F. et al. Do baseline glucocorticoids predict fitness? Trends in Ecology and Evolution 24, 634–642 (2009).
Boonstra, R., Hik, D. et al. The impact of predator-induced stress on the snowshore hare cycle. Ecological Monographs 68, 371–394 (1999).
Boonstra, R. et al. Plasma DHEA leves in wild, territorial red squirrels: season variation and effects of ACTH. General and Comparative Endocrinology 158, 61–67 (2008).
Carere, C. et al. Fecal corticosteroids in a territorial bird selected for different personalities: daily rhythm and the response to social stress. Hormones and Behavior 43, 540–548 (2003).
Cooper, W. E. Tradeoffs between courtship, fighting, and antipredatory behavior by a lizard, Eumece laticeps. Behavioral Ecology and Sociobiology 47, 54–59 (1999).
Cornil, C. A. et al. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research 166, 110–123 (2006).
Crawley, J. N. What's Wrong with my Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd ed. Hoboken, NJ: Wiley, 2007.
Cross, E. & Roselli, C. E. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology 276, R1346–R1350 (1999).
Gutzler, S. J. et al. Photoperiodic regulation of adrenal hormone secretion and aggression in female Syrian hamsters. Hormones and Behavior 56, 481–489 (2009).
Jasnow, A. M. et al. Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus). Hormones and Behavior 42, 13–20 (2002).
Breen, K. M. Karsch, F. J. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 145, 692–698 (2004).
Kobayashi, M. et al. Gonadotropin surge during spawning in male goldfish. General and Comparative Endocrinology 62, 70–79 (1986).
Koolhaas, J. M. et al. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews 23, 925–935 (1999).
Lord, L. D. et al. Rapid steroid influences on visually guided sexual behavior in male goldfish. Hormones and Behavior 56, 519–526 (2009).
Marler, C. A. et al. Increased energy-expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behavioral Ecology and Sociobiology 37, 225–231 (1995).
McEwen, B. S. & Wingfield, J. C. The concept of allostasis in biology and biomedicine. Hormones and Behavior 43, 2–15 (2003).
Mikics, E. et al. Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Hormones and Behavior 48, 152–162 (2005).
Mills, S. C. et al. Fitness trade-offs mediated by immunosuppression in a small mammal. Evolution 64, 166–179 (2010).
Moore, F. L. & Miller, L. J. Stress-induced inhibition of sexual behavior: corticosterone inhibits courtship behaviors of a male amphibian (Taricha granulosa). Hormones and Behavior 18, 400–410 (1984).
Orchinik, M., Murray, T. F. et al. A corticosteroid receptor in neuronal membranes. Science 252: 1848-51 (1991).
Orchinik, M., Murray, T. F. et al. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proceedings of the National Acadamey of Sciences USA 89,3830-4 (1992).
Pfaff, D. W. & Levine, J. E. Reconciling molecular neuroendocrine signals and the scientists who study them. Frontiers in Neuroendocrinology 29,167–168 (2008).
Pradhan, D. S. et al. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Hormones and Behavior 57, 381–389 (2010).
Remage-Healey, L. & Bass, A. H. From social behavior to neural circuitry: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Hormones and Behavior 50, 432–441 (2006).
Remage-Healey, L. et al. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience 11, 1327–1334 (2008).
Sapolsky, R. M. et al. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21, 55–89 (2000).
Scotti, M. A. et al. Aggressive encounters differentially affect serum dehydroepiandrosterone and testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Hormones and Behavior 56, 376–381 (2009).
Sih, A. et al. Behavioral syndromes: an integrative overview. Quarterly Review of Biology. 79, 241–277 (2004).
Soma, K. K. et al. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. Journal of Comparative Physiology 186, 759–769 (2000a).
Soma, K. K. et al. Oestrogen regulates male aggression in the non-breeding season. Proceedings of the Royal Society Londond Series B Biological Sciences 267, 1089–1096 (2000b).
Soma, K. K. & Wingfield, J. C. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Hormones and Behavior 123, 144–155 (2001).
Trainor, B. C. et al. Photoperiod reverses the effects of estrogens on male aggression via genomic and non-genomic pathways. Proceedings of the National Academy of Sciences USA 104, 9840–9845 (2007).
Trainor, B. C. et al. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice Hormones and Behavior 53, 192–199 (2008).
Valenstein, E. S. & Young, W. C. An experimental factor influencing the effectiveness of testosterone proprionate in eliciting sexual behavior in male guinea pigs. Endocrinology 56, 173–185 (1955).
Vasudevan, N. & Pfaff, D. W. Membrane initiated actions of estrogens in neuroendocrinology: emerging principles. Endocrine Reviews 28, 1–19 (2006).
Walters, M. J. & Harding, C. F. The effects of an aromatization inhibitor on the reproductive behavior of male zebra finches. Hormones and Behavior 22, 207–218 (1988).































