« Prev Next »

Aristotle wrote, around 350 BC, “teeth have one invariable office, namely the reduction of food.” Because mammals are warm blooded, they require fuel to heat their bodies and must squeeze as much energy as possible from the foods they eat. Teeth and chewing help because fractured and fragmented foods have more surface area on which digestive enzymes can act, and plant cell walls or insect exoskeletons that have been ruptured by teeth provide access to nutrients that would otherwise pass through the gut undigested. Teeth can thus be thought of as tools for accessing the energy and other nutrients in foods. And, because different foods have different fracture properties, they require different tools for the job. This entry discusses the relationships between tooth form and function, with an eye toward the fracture properties of foods that primates are adapted to eat. If we can understand these relationships, we can use fossil teeth to reconstruct the diets of our early hominin ancestors and other extinct primates.
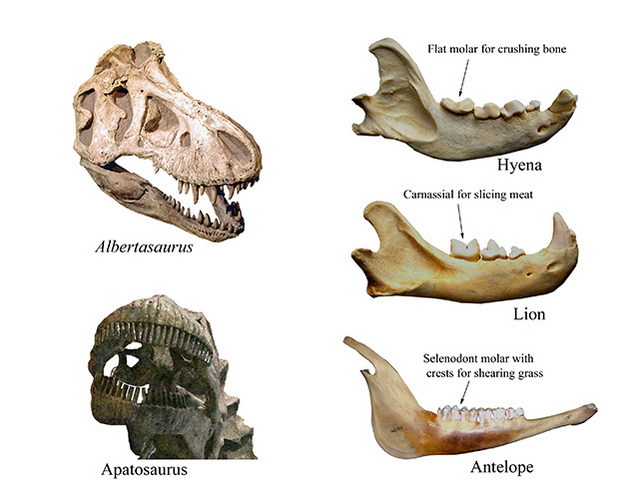
As any five-year-old child familiar with dinosaurs seems to know intuitively, animals with differently shaped teeth eat different things (Figure 1). While tyrannosaurid teeth are sharp and recurved, presumably for capturing and killing animals and/or ripping flesh from their carcasses, apatosaurids have small, peg-like teeth, more likely used for cropping vegetation. And, the tooth form-function relationship is just as important for mammals - albeit more complex given the need for precise occlusion between opposing teeth. Gazelles, for example, have fairly flat occlusal (biting) surfaces with ridges of hard enamel alternating with softer dentin to form roughened areas for grinding tough vegetation, such as grass. Lions in contrast, have specialized cheek teeth called carnassials, with sharp, v-shaped blades for slicing meat and sinew, and hyenas have blunter, thickly enameled teeth for crushing bone and other hard items. Many more details are provided in Ungar (2010).
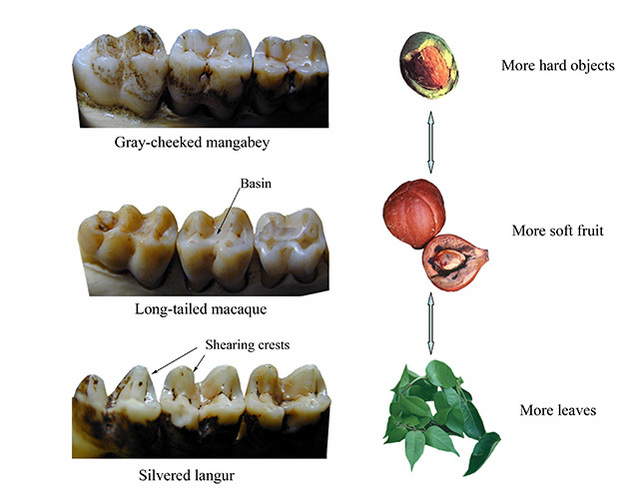
More closely related mammals also vary in tooth form depending on diet, albeit in subtler ways; such is the case with primates (Figure 2). Folivorous langurs have cheek teeth with taller cusps and longer shearing blades than do hard-object-feeding mangabeys. Frugivores, and those with broad diets, are intermediate in occlusal relief and crest length. In order to understand how such differences in tooth form relate to diet in primates, we must learn something about how foods defend themselves from fracture, and how teeth overcome those defenses without themselves being broken in the process. This is the domain of food-fracture mechanics, as described in detail by Lucas (2004; see also Ungar and Lucas, 2010).
FOOD FRACTURE PROPERTIES AND TEETH AS TOOLS
Most plant parts and animals have not evolved to be eaten (with a few exceptions, such as primate-dispersed angiosperm fruits). They are typically protected from being broken by either stress-limited or displacement-limited defenses. Stress-limited foods include hard-brittle items, such as some nuts and palm fronds, which require substantial stress (force per unit area) to initiate a crack, but once a crack starts, it is easily propagated. Displacement-limited foods, on the other hand, are typically tough items that may require little work to start a crack, but substantial energy to spread the crack through. Examples include raw meat and many mature leaves. This dichotomy is clearly an over-simplification, but it serves us well for understanding the challenges that teeth face during chewing.
We can start with stress-limited foods. We typically break hard-brittle items by crushing them between opposing teeth (think of a nutcracker). There is something counterintuitive about this though, because cracks tend to close with compression; they are spread when pulled apart, which requires tension. In fact, tension does usually occur, but perpendicular to the compressing force applied (try squeezing a walnut and watch what happens); and, because stress-limited foods tend to be inelastic, they fracture or fail without much deformation. So, what kinds of teeth are best suited for fracturing hard-brittle foods? Because stress equals force per unit area, the goal is to minimize the area of contact between the tooth and food item. A hemispheric cusp fit into a deep basin (or between staggered opposing cusps) works well to concentrate forces, keep food items in place, and keep the tooth itself from breaking. Thick enamel and complex enamel microstructure (see below) also help protect a tooth from breaking as high forces pass through them.
Displacement-limited foods impose different challenges on teeth. Since tough items tend not to be hard, initiating a crack is usually less of a problem than propagating it. Fracturing such foods can require constant work. A good tool in this case is a wedge; it creates tension at the tip of an advancing crack, spreading it further as the wedge itself advances (think of splitting a log). Opposing blades or crests that slide past one another (as with shears or scissors) work this way. And, there is little need to worry about breaking thin blades, as displacement-limited foods tend to be elastic; they deform and spread around the tooth in compression, effectively protecting it from fracture. In fact, many mammals adapted to consume tough foods have thin tooth enamel, which wears through to the underlying dentin in places. Sharp edges form where the harder enamel meets the softer dentin on the surface, which further facilitates fracture of foods (see below).
DENTAL FUNCTIONAL MORPHOLOGY
An understanding of relationships between tooth form and function requires a way of comparing dental morphology between species with different diets. Researchers have developed several approaches to quantifying functional aspects of occlusal shape in primates.
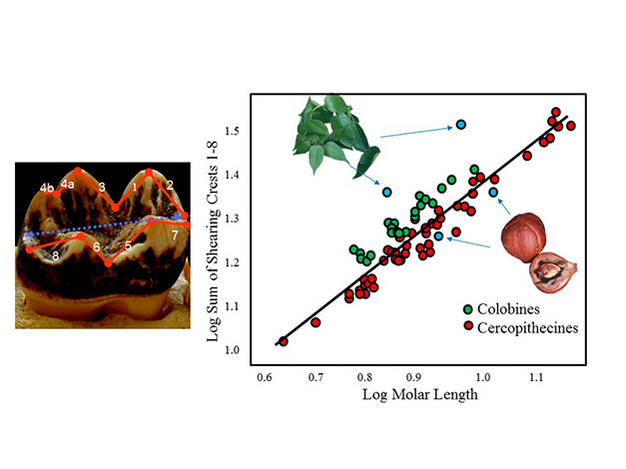
The traditional approach has been the study of shearing quotients (Kay, 1984). This involves calculation of the summed lengths of crests running up and over individual cusps relative to the mesiodistal length of the tooth as a whole (Figure 3). The longer the crests (i.e., taller or sharper the cusps), the higher the shearing quotient (SQ). Among closely related primates, folivores have higher SQs than frugivores, and among fruit-eaters those that consume hard objects have the lowest SQ values. This fits well with our predictions for occlusal morphology given the fracture properties of leaves, fruit flesh, and hard objects. We should therefore be able to infer diets of fossil species based on where they fall on a plot of molar-crest length against tooth length for living primates with known food preferences (Figure 3).
Shearing quotient studies work well with unworn molars, but it becomes difficult to measure crest lengths when these teeth wear down. That said, most fossil teeth recovered are worn. Moreover, limiting our studies to unworn specimens gives us only part of the picture because nature should select not only for unworn dental forms, but also for teeth that wear in ways that make or keep them functionally efficient. Dental topographic analysis was developed with this in mind (e.g., Ungar and M’Kirera, 2003). In these studies, occlusal surfaces are represented as clouds of points using a laser scanner or other device, and these point clouds are imported into Geographic Information Systems (GIS) software packages. Tooth surfaces are then modeled as landscapes - cusps become mountains, fissures become valleys, and so on. Average surface slope, relief, angularity, and other attributes can be measured and compared both between species, and within species between individuals with differently worn teeth. And, at given stages of wear, dental topography has been shown to track diet well (e.g., folivores at a particular stage of wear have more sloping occlusal surfaces with more topographic relief than do frugivores at the same stage). As with SQ studies, data for fossil species can be compared directly with those for closely related living primates with known diets.
FUTURE DIRECTIONS
Work is progressing on understanding dental functional morphology and biomechanics as they relate to food fracture properties. Studies of enamel thickness across the crown, as well as enamel chemistry and microstructure are especially promising (see Ungar, 2010).
Teeth must transmit the forces needed to break food items without themselves being broken. This is especially challenging for hard-object feeders, which generate high stresses to crack food items. Because cheek teeth are effectively bi-layered (with a stiffer, harder enamel cap overlaying softer, more pliable dentin), thicker enamel should prevent a crown from flexing and cracking in a high stress environment (Lucas et al., 2008). The chemistry and microstructure of the enamel itself can also affect the strength of the crown, and its resistance to breakage. For example, primate enamel varies in its degree of mineralization, and therefore its hardness and stiffness, from one part of the crown to another (e.g., Darnell et al., 2010). Further, because enamel is formed in rows of long, parallel rods or prisms, the pattern by which these undulate and interweave (called decussation) can help slow or stop the spread of cracks through the enamel layer (see Maas and Dumont, 1999 for review). New technologies, such as phase contrast x-ray synchrotron microtopography are beginning to provide incredible detail on the microscopic layout of dental enamel (see Smith and Tafforeau, 2008). This level of detail will undoubtedly lead to better understandings of the ways primate teeth have evolved to resist breakage and, ultimately, the fracture properties of the foods a primate species has evolved to eat.
Tooth enamel may also provide a mechanism to guide wear. Many mammals have what has been termed secondary morphology: their teeth function most efficiently when worn (Fortelius, 1985). Some mammals even erupt and begin to grind their teeth in the womb, so that they are “cut” and ready to go at birth. The spatial relationship between the enamel cap and underlying dentin horns may be especially important for primates in this regard. Softer dentin will be exposed with wear more quickly on those parts of the crown with thinner enamel, leaving sharp edges where the two tissue types meet at the surface. Thus, the distribution of enamel over the crown can literally sculpt the surface with wear; and again, new technologies, such as x-ray microcomputed tomography, are allowing us to map these distributions (see Smith and Tafforeau, 2008). Such studies will surely provide even more insights into primate dental functional morphology.
Glossary
Basin (tooth) - depressed area or hollow on the occlusal surface, often enclosed or surrounded by cusps or crests.
Carnassial - a specialized cheek tooth (last upper premolar and first lower molar in cats and dogs) with long, blade-like crests for slicing meat and other tough items
Cercopithecine - a member of the subfamily of Old World monkeys characterized by adaptations to consume soft, fleshy fruits
Cheek teeth - premolar and molar teeth (those behind the canines and adjacent to the cheeks)
Colobine - a member of the subfamily of Old World monkeys characterized by adaptations to consume leaves and seeds
Crest (tooth) - an elongated ridge of enamel on the occlusal surface, usually connecting two cusps
Crown (tooth) - the exposed portion of the tooth, usually covered in enamel
Decussation - the pattern by which microscopic rods or prisms of enamel cross and interweave as they are laid down during dental development
Dental topographic analysis - a method for analyzing teeth as if they were topographic surfaces. This typically involves generating 3D point clouds for a given tooth surface, and modeling them using a geographic information system.
Dentin - the mineralized hard tissue (approximately 70% hydroxyapatite) that comprises most of the root and the crown underlying the enamel cap
Displacement-limited - referring to a tough food item, one that resists breakage by resisting the spread of a crack
Enamel (tooth) - the mineralized hard tissue (approximately 97% hydroxyapatite) that comprises the outer cap of the tooth crown for most mammals
Enamel chemistry - the chemical composition of enamel. The degree of mineralization of enamel, for example, affects it hardness, and the isotopic composition of carbon within the enamel tells us whether the animal ate tropical grasses (with high levels of carbon-13) or most other plants.
Enamel microstructure - the microscopic structure of enamel, usually referring to the layout of rods or prisms that form the enamel layer of a tooth
Folivorous - referring to animals that consumes leaves
Fracture properties - characteristics describing the resistance of an object to the start or spread of a crack
Frugivores -animals that consumes fruit, especially the soft, fleshy parts
Mesiodistal - the axis of a tooth that runs from the front of the mouth to the back
Molars -back teeth. In mammals, these are typically not replaced, but added behind the final premolar as the jaw grows long enough to accommodate them.
Occlusion - The interdigitation or fitting together of opposing upper and lower teeth
Phase contrast x-ray synchrotron microtomography - a technology that uses x-rays with high beam intensity and contrast to generate extremely high resolution (micron to submicron) 3D images of the internal structure of tooth enamel and other tissues
Shearing blades - sharp, elongated ridges or crests of enamel on the occlusal surface running from the front to the back of the tooth up and over the cusp tips
Shearing quotient - a measure of the ratio of the lengths of shearing blades on a tooth to the length of the tooth. The higher the shearing quotient (SQ), the better suited a tooth is to shearing tough foods.
Stress-limited - referring to a hard food item, one that resists breakage by resisting the initiation of a crack
X-ray microcomputed tomography - a method using x-rays to create cross-sections of 3D objects at high resolutions (typically in the range of 10s of microns)
References and Recommended Reading
Darnell, L.A., Teaford, M.F. et al. Variations in the mechanical properties of Alouatta palliata molar enamel. American Journal of Physical Anthropology 141, 7-15 (2010).
Fortelius, M. Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zoologica Fennica 180, 1-76 (1985).
Kay, R.F. On the use of anatomical features to infer foraging behavior in extinct primates. In Adaptations for Foraging in Nonhuman Primates: Contributions to an Organismal Biology of Prosimians, Monkeys, and Apes. eds. Rodman, P.S. & Cant, J.G.H. (New York: Columbia University Press 1984) 21-53.
Lucas, P.W. Dental Functional Morphology: How Teeth Work. New York: Cambridge University Press (2004).
Lucas, P.W., Constantino, P. et al. Dental enamel as a dietary indicator in mammals. Bioessays 30, 374-358 (2008).
Maas, M.C. & Dumont, E.R. Built to last: the structure, function, and evolution of primate dental enamel. Evolutionary Anthropology 8, 133-152 (1999).
Smith, T.M. & Tafforeau, P. New visions of dental tissue research: tooth development, chemistry, and structure. Evolutionary Anthropology 17, 213-226 (2008).
Ungar, P.S. Dental allometry, morphology, and wear as evidence of diet in fossil primates. Evolutionary Anthropology 6, 205-217 (1998).
Ungar, P.S. Mammal Teeth: Origin, Evolution, and Diversity. Baltimore: The Johns Hopkins University Press (2010).
Ungar, P.S. & Lucas, P.W. Tooth form and function in biological anthropology. In A Companion to Biological Anthropology. ed. Larsen, C.S. (Malden, MA: Wiley-Blackwell 2010) 516-529.
Ungar, P.S. & M’Kirera, F. A solution to the worn tooth conundrum in primate functional anatomy. Proceedings of the National Academy of Sciences of the United States of America 100, 3874-3877 (2003).