« Prev Next »
Introduction
Historically, primate cranial variation was conceptualized in terms of a series of progressive evolutionary "tendencies" such as enlargement of the brain, decreased reliance on olfaction, increased visual acuity, reduction of the jaws and dentition, and assumption of more orthograde (upright) head postures (e.g., Le Gros Clark, 1959; Biegert, 1963). These tendencies or trends delineated a morphological continuum extending from small-brained, snouty "lower primates" (prosimians) through more "advanced" anthropoids (monkeys and apes) and culminating with large-brained, small-faced Homo sapiens.
Over time, changes in systematic philosophies and advances in analytical methods have reshaped our thinking about primate diversity. Since the advent of multivariate morphometric analysis, cranial diversity is more commonly conceptualized as a multidimensional "morphospace" (Figure 1), within which species can be mapped relative to axes of morphological variation corresponding to features such as endocranial volume and facial length. Major axes of primate cranial variation often reflect reorganizations of cranial structure that define important evolutionary events such as the strepsirrhine-haplorhine divergence or the origin of modern apes. For this reason, the morphological patterns that these axes summarize often mirror the classical evolutionary "trends" described by early primatologists (e.g., Fleagle et al., 2010). Click on the animation below for a 360° view of the 3D morphospace shown in Figure 1a.
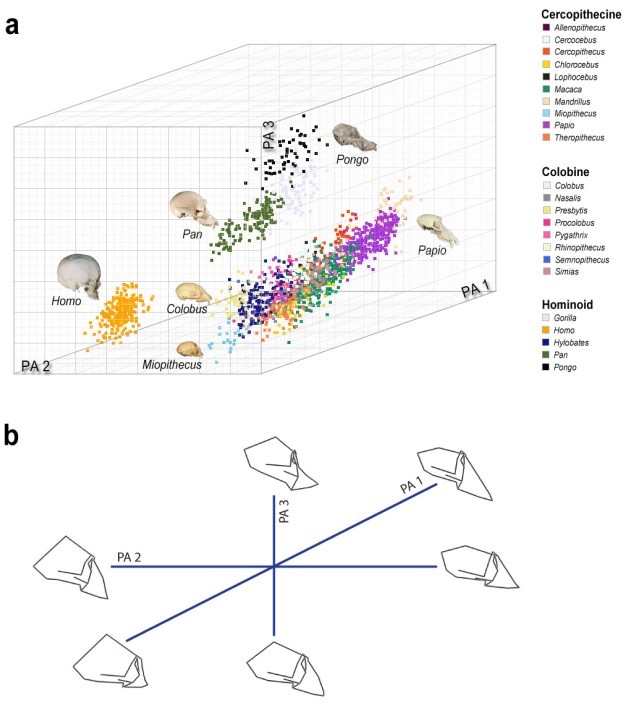
Superimposed on phylogenetic differences among major primate groups, there is considerable variation within groups relating to species' social and ecological behavioral patterns. For reasons noted above, this variation is not random. Morphological integration of cranial form requires that certain traits covary in predictable patterns, which function as evolutionary paths of least resistance for natural selection to follow (Schluter, 1996). Likewise, not all morphological combinations are functionally viable, leaving regions of the theoretical morphospace unoccupied. Using these observed patterns of morphological covariation, primate morphologists attempt to understand the determinants of cranial variation and to reconstruct the developmental, adaptive, and evolutionary bases of primate cranial diversity.
Cranial Size and Shape
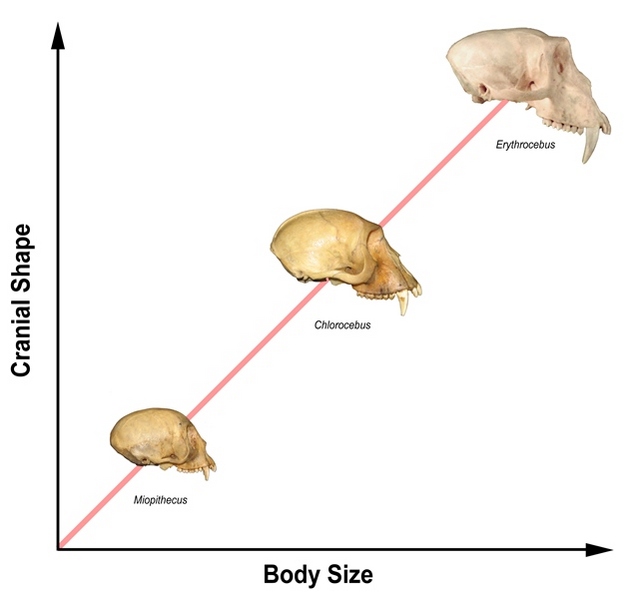
Next to phylogeny, perhaps the most pervasive influence on primate cranial form is body size. In primates, as in other mammals, the physical dimensions of the major cranial regions exhibit faster or slower rates of increase (positive versus negative allometry) as a function of increasing body size (Huxley, 1932). Brain volume and, consequently, neurocranial dimensions scale with negative allometry relative to body size, while facial dimensions (especially length) scale positively (Figures 1 and 2). Accordingly, small primates have proportionately larger neurocrania and shorter faces than large primates (Gould, 1975; Martin, 1990). Size-related shape changes have a cascade effect, as spatial relationships among the cranium's functional subunits adjust to accommodate altered proportions, making body size a major determinant of cranial form. The role of body-size variation as a "line of least evolutionary resistance" has been documented in both Old and New World monkeys (Figure 2). In these cases, diversification of cranial form appears to be largely an indirect consequence of selection on body size, and levels of shape diversity reflect both the magnitude of size disparities and the strength of allometric relationships in different groups (Schluter, 1996; Ravosa & Profant, 2000; Marroig & Cheverud, 2005).
Cranial Base Flexion
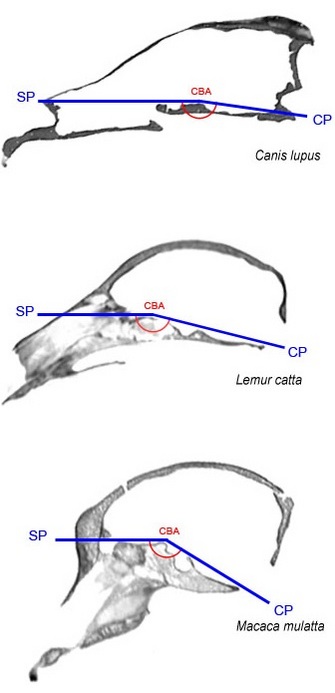
Primates are distinguished from other mammals by increased flexion of the cranial base in the midsagittal plane (Huxley, 1863, Le Gros Clark, 1959, Biegert, 1963). Basicranial flexion is defined by a decrease in the cranial base angle (CBA) formed between the posterior (postsellar) and anterior (presellar) segments of the base (Figure 3). In general, smaller CBAs distinguish primates from nonprimates, haplorhines from strepsirrhines, and hominins from other apes. The "spatial-packing hypothesis" of Biegert (1963) attributed increased basicranial flexion in primates to their large brains, and subsequent studies have confirmed that relative brain size (encephalization) is the primary influence on cranial base flexion (Ross & Ravosa, 1993; Lieberman et al., 2000). Across all primates, CBA is inversely correlated with encephalization; thus, relatively large-brained primates have more flexed crania, while relatively small-brained primates have more extended crania. However, the strength of this correlation differs among primate groups, and other factors, particularly facial size, contribute to variation in basicranial flexion (Ross & Ravosa, 1993; Lieberman et al., 2000, 2008; Bastir et al., 2010). Cranial base angle, in turn, determines spatial relationships among major functional units, including the braincase, orbits, face, and pharynx, giving basicranial flexion a pivotal role in cranial integration (Lieberman et al., 2000).
Orbital Size and Orientation
Acute binocular vision is a defining character of modern primates and is central to recent hypotheses for primate origins, most notably the visual predation hypothesis (Cartmill, 1972; Sussman, 1991; Ravosa & Savakova, 2004; Ross & Kirk, 2007; Heesy, 2008). Many aspects of primates' cranial morphology are directly related to their large "forward-facing" eyes. Simultaneously, differences in orbital size, orientation, and bony structure signal phylogenetic and ecological divergences between primate taxa (Cartmill, 1972; Kay & Kirk, 2000; Heesy, 2005; Kirk, 2006; Ross & Kirk, 2007). As might be expected, eye size is the principal influence on both orbital volume and orbital aperture diameter (Schultz, 1940; Kirk, 2006), but relationships among body size, eye size, and orbital size are complex. Both eye size and orbital size scale with negative allometry relative to body size; moreover, eye size scales negatively relative to orbital size (Schultz, 1940; Martin, 1990). Accordingly, small primates have relatively large eyes and orbits (Figure 4a-d), while large primates have relatively small orbits that constitute a smaller proportion of facial volume. Activity pattern also influences eye and orbital size. Nocturnal primates have relatively larger orbital apertures than diurnal primates, with cathemeral taxa having intermediate apertures (Martin, 1990; Ross, 1995a; Kay & Kirk, 2000; Kirk, 2006). This generalization holds despite differences in eye structure and orbital morphology that complicate comparisons between strepsirrhines and haplorhines (Kirk, 2006; Ross & Kirk, 2007). In addition, nocturnal strepsirrhines that prey on small animals have relatively larger eyes and orbits than nonpredatory strepsirrhines, irrespective of activity pattern (Kirk, 2006). Among haplorhines, whose eyes lack features associated with low-light vision (Kay & Kirk, 2000; Ross & Kirk, 2007), nocturnal species such as tarsiers and owl monkeys have evolved dramatically enlarged eyeballs, which allow relatively high visual acuity under low-light conditions (Kirk, 2006).
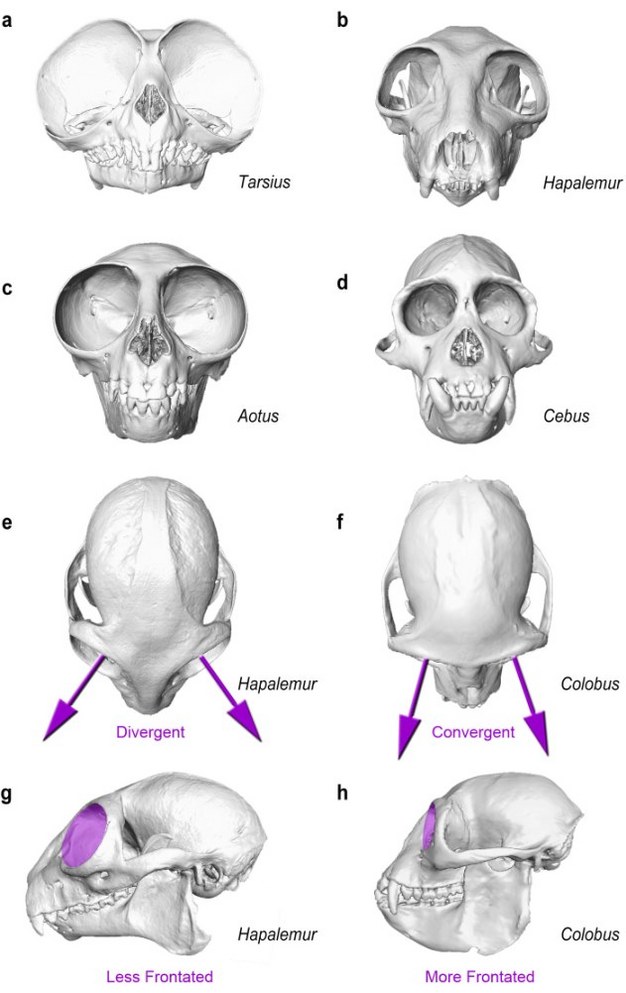
Orbital orientation (Figure 4e-h) is described in terms of convergence, the extent to which the orbits face in the same direction, and frontation, the vertical orientation of the orbital aperture relative to the neurocranium and/or lower face (Cartmill, 1972; Heesy, 2008). Relationships among orbital size, convergence, and frontation vary with cranial size and differ between strepsirrhines and haplorhines (Ross, 1995a). Across all primates, convergence is negatively correlated with relative orbital size, and larger primates have higher convergence; however, small, nocturnal primates with large, strongly convergent orbits (e.g., tarsiers and lorises) are notable exceptions to this rule (Lieberman et al., 2000). Olfactory reduction in haplorhines is associated with extreme convergence relative to strepsirrhines of similar orbital size. Haplorhine orbits are also more vertical, on average, than those of strepsirrhines with equivalent convergence (Ross, 1995a). Among strepsirrhines, frontation is inversely correlated with convergence, most notably in lorisids, whose large, unfrontated orbits converge "upwards," superior to the olfactory region (Ross, 1995a). In haplorhines, approximation of the orbits inferior to the olfactory tract integrates them structurally with the anterior cranial base. Accordingly, frontation increases with increasing basicranial flexion, and the anthropoid combination of high orbital convergence and high frontation is unique among mammals (Ross & Ravosa, 1993, Heesy, 2008). Extreme convergence and frontation account for another hallmark of the haplorhine orbit, the expansion of the primitive postorbital bar into a bony septum (incomplete in tarsiers). This postorbital closure is hypothesized to have arisen in small-bodied, ancestral haplorhines to buffer repositioned orbital contents from adjacent masticatory muscles, which would otherwise interfere with optical alignment and reduce visual acuity during feeding (Ross & Ravosa, 1993; Ross, 1995b; Ravosa et al., 2000; Heesy, 2005).
Facial Prognathism and Kyphosis
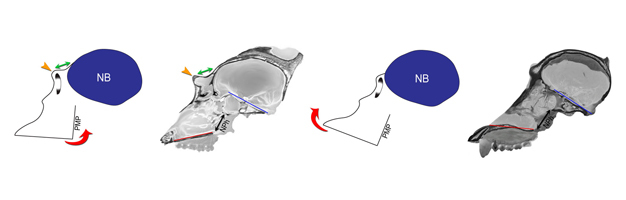
Angular orientation of the face relative to the neurobasicranium, or kyphosis, is most strongly influenced by cranial base form. In anthropoids, the upper face (orbital region) is tightly integrated with the anterior cranial base, which contributes to the orbital roof. Structural integration of the midface (nasal region) is comparatively weaker, although its border with the cranial base (the posterior maxillary plane) maintains a 90° angle with the orbit's horizontal axis (Enlow & McNamara, 1973; Lieberman et al., 2000). The lower face (palate) integrates with the cranial base only indirectly, maintaining a parallel relationship with the orbits. Together, the upper face and posterior maxillary plane form a structural block that rotates ventrally and posteriorly as the cranial base flexes (Figure 5a), decreasing the angle between the base and palate and bringing the upper face beneath the braincase, a condition called klinorhynchy (Lieberman et al., 2000). The position of the nasopharynx between the palate and cranial base (Figure 5a,c) limits kyphosis, indirectly constraining cranial base angle, especially in relatively long-faced species (Ross & Henneberg, 1995; Lieberman et al., 2000). This constraint has been circumvented in great apes (Figure 1b, PA 3), which have more dorsally oriented (airorhynch) faces than expected for their strongly flexed cranial bases (Shea, 1988; Lieberman et al., 2000). Additionally, primates with enlarged laryngeal sacs, such as male howler monkeys and orangutans (Figure 5d), are more airorhynch than related species lacking these specializations (Biegert, 1963).
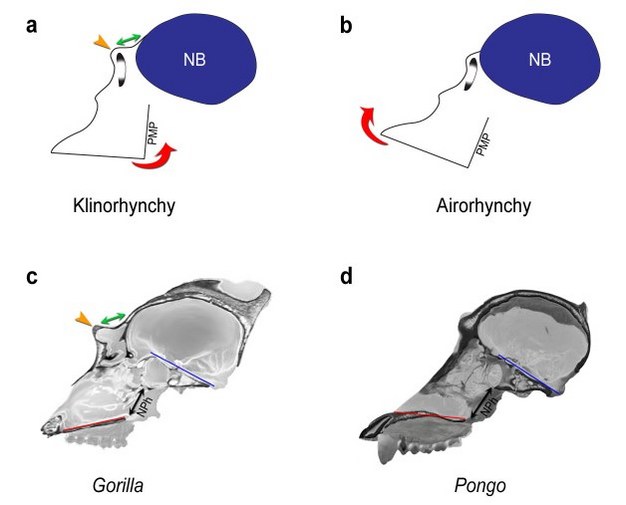
The relationship between kyphosis and cranial base angle affects other aspects of facial form. For example, increased facial projection in large-bodied anthropoids is associated with spatial separation between the anterior braincase and orbits and formation of a shelf-like supraorbital torus or browridge (Shea, 1985a,b; Ravosa, 1991; Lieberman, 2000). In apes and Old World monkeys, klinorhynchy enhances this "neuro-orbital disjunction" (Figure 5a,c), resulting in a relatively longer browridge (Moss & Young, 1960; Ravosa, 1991; Lieberman, 2000). Conversely, the dorsal orientation (airorhynchy) of the orangutan face reduces neuro-orbital disjunction (Figure 5b,d), resulting in a shorter browridge relative to cranial size (Shea, 1985a, 1988; Lieberman, 2000).
Summary
This article summarizes important axes of primate cranial variation and explores evolutionary, developmental, and functional influences that contribute to primate cranial diversity. This review necessarily lacks the detail and nuance of the sources on which it draws. Readers are encouraged to use the references cited as a launching point for further independent exploration of the primate cranial morphospace.
Glossary
Activity pattern - an animal's habitual times of activity and rest throughout the daily cycle
Airorhynchy - pattern of facial kyphosis in which the face is dorsally oriented relative to the neurobasicranium and the face lies anterior to the anterior cranial base
Allometry - changes in the relative proportions (i.e., shape) of an organism that are correlated with increase or decrease in body size
Anthropoid - belonging to or characteristic of the group of primates that includes monkeys and apes. Modern anthropoids are classified to the primate infraorders Platyrrhini (New World monkeys) and Catarrhini (Old World monkeys, apes, and humans).
Basicranial flexion - the angular relationship between the anterior and posterior portions of the cranial base in the midsagittal plane
Browridge - see supraorbital torus
Canine sexual dimorphism - differences between males and females of a species in relative canine size and/or projection
Cathemeral - active intermittently throughout the 24-hour cycle during periods of both daylight and darkness
Convergence - the extent to which the orbits face in the same direction
Cranial base angle - any of several defined angles that measure basicranial flexion
Diurnal - active primarily during daylight hours
Dorsal - located toward the back or posterior surface of a structure or organism. The dorsal surface of the cranium includes the superior and posterior aspects of the braincase and, in prognathic species, the superior surface of face.
Encephalization - the relationship between brain size and overall body size. Animals with relatively large brains are said to be more encephalized.
Facial prognathism - forward protrusion of the face relative to the neurocranium. Prognathism is influenced by relative facial length, the relative distance between the face and the neurocranium (facial projection), and basicranial flexion.
Frontation - term describing the vertical orientation of the orbital aperture relative to the neurocranium and/or palate.
Gape - measure of the extent to which the lower jaw (mandible) can be opened. The maximum gape an animal can achieve is determined by its relative jaw length, jaw joint structure, and masticatory muscle structure.
Haplorhine - belonging to or characteristic of infraorder Haplorhini, the group of primates including tarsiers, monkeys, and apes
Hominin - belonging to or characteristic of the primate group including living and extinct species of genus Homo as well as other extinct species more closely related to Homo than to chimpanzees (genus Pan)
Klinorhynchy - pattern of facial kyphosis in which the face is ventrally oriented relative to the neurobasicranium and the face lies inferior to the anterior cranial base
Kyphosis - angular orientation of the face relative to the neurocranium and/or cranial base. The degree of facial kyphosis is described in terms of klinorhynchy (more ventral orientation) versus airorhynchy (more dorsal orientation).
Lorisid - belonging to or characteristic of the strepsirrhine family Lorisidae, which includes lorises and pottos
Mastication - the process of crushing and/or grinding food between the teeth
Masticatory muscles - a group of muscles whose principal function is to move the lower jaw (mandible) during ingestion and mastication of food
Midsagittal plane - a standard anatomical reference plane that divides the cranium into symmetric right and left halves
Morphological integration - developmental and/or functional association among morphological units such that changes in one region necessitate correlated changes in integrated regions
Morphometric - relating to the measurement of the form of biological organisms or their constituent parts
Morphospace - a quantitative and/or graphical representation of biological variation across two or more dimensions or characters. A theoretical morphospace encompasses all possible unique combinations of the attributes under consideration.
Nasopharynx - the portion of the respiratory tract located immediately inferior to the cranial base and posterior to the nasal cavity
Neurobasicranium - the posterior portion of the cranium including both the neurocranium (braincase) and cranial base
Neuro-orbital disjunction - term describing the relative degree of spatial separation between the anterior neurocranium and upper face, particularly the orbits
Nocturnal - active primarily during hours of darkness
Olfaction - the sense of smell
Orthognathic - term describing a relative absence of facial projection, resulting in a vertical facial profile
Phylogenetic - relating to or resulting from the evolutionary history of a group of organisms
Phylogeny - the evolutionary history of a group of organisms or the graphical representation of that history, usually as a branching diagram or tree
Posterior maxillary plane - anatomical reference plane marking the boundary between the midface (nasal region) anteriorly and the neurocranium posteriorly
Postorbital bar - bony arch that bridges the lateral orbit completing the circular orbital aperture. In primates, the postorbital bar is formed by projections from the frontal and zygomatic bones.
Postorbital closure - used in its strictest sense, the presence of a complete bony septum separating the orbit from the temporal region. In anthropoids, the postorbital septum includes portions of the frontal, zygomatic, and sphenoid bones.
Prosimian - belonging to or characteristic of the group of primates that includes lemurs, lorises, galagos, and tarsiers. All prosimians except tarsiers (which are more closely related to monkeys and apes) are classified to the primate suborder Strepsirrhini.
Radiation - process of diversification within a rapidly evolving lineage or the resulting group of species
Strepsirrhine - belonging to or characteristic of infraorder Strepsirrhini, the group of primates including lemurs, lorises, and galagos but excluding tarsiers
Supraorbital torus - a shelf of bone located superior to the orbits and anterior to the braincase
Ventral - located toward the abdominal surface of a structure or organism. The ventral surface of the cranium includes both the cranial base and the palate.
Visual predation hypothesis - a scientific hypothesis developed by Cartmill (1972) linking shared primate traits, such as orbital convergence and grasping hands, to predation on insects and other small prey. More recent versions of this hypothesis emphasize the importance of nocturnal visual predation to the evolution of the primate visual system.
References and Recommended Reading
Bastir, M. et al. Effects of brain and facial size on basicranial form in human and primate evolution. Journal of Human Evolution 58, 424-431 (2010).
Biegert, J. The evaluation of characteristics of the skull, hands, and feet for primate taxonomy. In Classification and Human Evolution. Ed. S. L. Washburn (Chicago: Aldine, 1963) 116-145.
Cartmill, M. Arboreal adaptations and the origin of the order Primates. In The Functional and Evolutionary Biology of Primates. Ed. R. Tuttle (Chicago: Aldine, 1972) 97-122.
Enlow, D. H. & McNamara, J. A. The neurocranial basis for facial form and pattern. Angle Orthodontist 43, 256-270 (1973).
Fleagle, J. G. et al. Primate cranial diversity. American Journal of Physical Anthropology 142, 565-578 (2010).
Gould, S. J. Allometry in primates, with emphasis on scaling and the evolution of the brain. In Approaches to Primate Paleobiology. Ed. F. S. Szalay (Basel: Karger, 1975) 244-292.
Heesy, C. P. Function of the mammalian postorbital bar. Journal of Morphology 264, 363-380 (2005).
Heesy, C. P. Ecomorphology of orbit orientation and the adaptive significance of binocular vision in primates and other mammals. Brain, Behavior and Evolution 71, 54-67 (2008).
Huxley, J. S. Problems of Relative Growth. London: Methuen (1932).
Huxley, T. H. Evidence as to Man's Place in Nature. London: Williams and Norgate (1863).
Kay, R. F. & Kirk, E. C. Osteological evidence for the evolution of activity pattern and visual acuity in primates. American Journal of Physical Anthropology 113, 235-262 (2000).
Kirk, E. C. Effects of activity pattern on eye size and orbital aperture size in primates. Journal of Human Evolution 51, 159-170 (2006).
Koyabu, D. B. & Endo, H. Craniodental mechanics and diet in Asian colobines: morphological evidence of mature seed predation and sclerocarpy. American Journal of Physical Anthropology 142, 137-148 (2010).
Le Gros Clark, W. E. L. The Antecedents of Man: An Introduction to the Evolution of the Primates. Edinburgh: Edinburgh University Press (1959).
Lieberman, D. E. Ontogeny, homology, and phylogeny in the hominid craniofacial skeleton: the problem of the browridge. In Development, Growth and Evolution. Eds. P. O'Higgins and M. Cohen (London: Linnean Society, 2000) 86-122.
Lieberman, D. E. et al. Spatial packing, cranial base angulation, and craniofacial shape variation in the mammalian skull: testing a new model using mice. Journal of Anatomy 212, 720-735 (2008).
Lieberman, D. E. et al. The primate cranial base: ontogeny, function, and integration. Yearbook of Physical Anthropology 43, 117-169 (2000).
Marroig, G. & Cheverud, J. M. Size as a line of least evolutionary resistance: diet and adaptive morphological radiation in New World monkeys. Evolution 59, 1128-1142 (2005).
Martin, R. D. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton, New Jersey: Princeton University Press (1990).
Moss, M. L. & Young, R. W. A functional approach to craniology. American Journal of Physical Anthropology 281-92 (1960).
Ravosa, M. J. Interspecific perspective on mechanical and nonmechanical models of primate circumorbital morphology. American Journal of Physical Anthropology 86, 369-396 (1991).
Ravosa, M. J. et al. Masticatory stress, orbital orientation and the evolution of the primate postorbital bar. Journal of Human Evolution 38, 667-693 (2000).
Ravosa, M. J. & Profant, L. P. Evolutionary morphology of the skull in Old World monkeys. In Old World Monkeys. eds. P. F. Whitehead and C. J. Jolly (Cambridge: Cambridge University Press, 2000) 237-268.
Ravosa, M. J. & Savakova, D. G. Euprimate origins: the eyes have it. Journal of Human Evolution 46, 357-364 (2004).
Ross, C. F. Allometric and functional influences on primate orbit orientation and the origins of the Anthropoidea. Journal of Human Evolution 29, 201-227 (1995a).
Ross, C. F. Muscular and osseous anatomy of the primate anterior temporal fossa and the functions of the postorbital septum. American Journal of Physical Anthropology 98, 275-306 (1995b).
Ross, C. F. & Henneberg, M. Basicranial flexion, relative brain size and facial kyphosis in Homo sapiens and some fossil hominids. American Journal of Physical Anthropology 98, 575-593 (1995).
Ross, C. F. & Kirk, E. C. Evolution of eye size and shape in primates. Journal of Human Evolution 52, 294-313 (2007).
Ross, C. F. & Ravosa, M. J. Basicranial flexion, relative brain size, and facial kyphosis in nonhuman primates. American Journal of Physical Anthropology 91, 305-324 (1993).
Schluter, D. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766-1774 (1996).
Schultz, A. The size of the orbit and of the eye in primates. American Journal of Physical Anthropology 26, 389-408 (1940).
Shea, B. T. On aspects of skull form in African apes and orangutans, with implications for hominoid evolution. American Journal of Physical Anthropology 68, 329-342 (1985a).
Shea, B. T. On skull form and the supraorbital torus in primates. Current Anthropology 27, 257-260 (1985b).
Shea, B. T. Phylogenetic aspects of skull form in the hominoid primates. In Orang-utan Biology. Ed. J. H. Schwartz (New York: Oxford University Press, 1988) 233-245.
Sussman, R. Primate origins and the evolution of angiosperms. American Journal of Primatology 23, 209-223 (1991).
Wright, B. Craniodental biomechanics and dietary toughness in the genus Cebus. Journal of Human Evolution 48, 473-92 (2005).