« Prev Next »
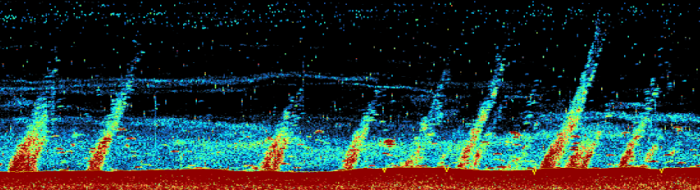
As the evidence for warming climate became better established in the latter part of the 20th century (IPCC 2001), some scientists raised the alarm that large quantities of methane (CH4) might be liberated by widespread destabilization of climate-sensitive gas hydrate deposits trapped in marine and permafrost-associated sediments (Bohannon 2008, Krey et al. 2009, Mascarelli 2009). Even if only a fraction of the liberated CH4 were to reach the atmosphere, the potency of CH4 as a greenhouse gas (GHG) and the persistence of its oxidative product (CO2) heightened concerns that gas hydrate dissociation could represent a slow tipping point (Archer et al. 2009) for Earth's contemporary period of climate change.
Methane Hydrate Primer
Methane hydrate is an ice-like substance formed when CH4 and water combine at low temperature (up to ~25ºC) and moderate pressure (greater than 3-5 MPa, which corresponds to combined water and sediment depths of 300 to 500 m). Globally, an estimated 99% of gas hydrates occurs in the sediments of marine continental margins at saturations as high as 20% to 80% in some lithologies; the remaining 1% is mostly associated with sediments in and beneath areas of high-latitude, continuous permafrost (McIver 1981, Collett et al. 2009). Nominally, methane hydrate concentrates CH4 by ~164 times on a volumetric basis compared to gas at standard pressure and temperature. Warming a small volume of gas hydrate could thus liberate large volumes of gas.
A challenge for assessing the impact of contemporary climate change on methane hydrates is continued uncertainty about the size of the global gas hydrate inventory and the portion of the inventory that is susceptible to climate warming. This paper addresses the latter issue, while the former remains under active debate. Dickens (2011) recently estimated 7x102 to 1.27x104 Gt carbon (Gt C) to be sequestered in marine gas hydrates alone, while Shakhova et al. (2010a) estimate 3.75x102 Gt C in methane hydrates just on the East Siberian Arctic shelf (ESAS). A conservative estimate (Boswell & Collett 2011) for the global gas hydrate inventory is ~1.8x103 Gt C, corresponding to CH4 volume of ~3.0x1015 m3 if CH4 density is taken as 0.717 kg/m3. In the unlikely event that 0.1% (1.8 Gt C) of this CH4 were instantaneously released to the atmosphere, CH4 concentrations would increase to ~2900 ppb from the 2005 value of ~1774 ppb (IPCC 2007).
Why Methane Matters
Concern about the long-term stability of global gas hydrate deposits is rooted in the potential impact that a large CH4 release might have on global climate. CH4 is ~20 times more potent than CO2 as a GHG, but it oxidizes to CO2 after about a decade in the atmosphere. In recent models, the longer-lived CO2 oxidation product (Archer et al. 2009), not the CH4 itself (e.g., Harvey & Huang 1992), is credited with causing most of the excess atmospheric warming that would follow large-scale dissociation of methane hydrates.
The present-day concentration of CH4 in the atmosphere is ~200 times lower than that of CO2, but CH4 concentrations have risen by ~150% since pre-industrial times, compared to only ~40% for CO2 (IPCC 2001). Rising atmospheric CH4 concentrations lead to more rapid depletion of the hydroxyl radicals needed for oxidation, longer CH4 residence times, and thus increased CH4-induced warming (Lelieveld et al. 1998). Present-day CH4 emissions are dominated by wetlands, ruminants, fossil fuel production, and rice cultivation (IPCC 2001), sources that fluctuate with season, human behavior, and other factors. The IPCC (2001, 2007) relies on estimates and models (Fung et al. 1991, Lelieveld et al. 1998, Wang et al. 2004) to attribute ~3.75x10-4 Gt C (IPCC 2001), or ~1% of annual global CH4 emissions, to dissociating gas hydrates. This estimate has never been validated, nor do any observational data unequivocally link specific CH4 emissions or regionally integrated CH4 fluxes to dissociating gas hydrates. Against the backdrop of a well-mixed atmosphere, strong annual fluctuations, and a significant increase in CH4 concentrations over the 20th century, detecting a CH4 signal that is directly attributable to dissociating gas hydrates may always remain challenging.
Gas Hydrates and Past Warming Events
Fate of Contemporary Methane Hydrates During Warming Climate
The susceptibility of gas hydrates to warming climate depends on the duration of the warming event, their depth beneath the seafloor or tundra surface, and the amount of warming required to heat sediments to the point of dissociating gas hydrates. A rudimentary estimate of the depth to which sediments are affected by an instantaneous, sustained temperature change DT in the overlying air or ocean waters can be made using the diffusive length scale 1 = √kt , which describes the depth (m) that 0.5 DT will propagate in elapsed time t (s). k denotes thermal diffusivity, which ranges from ~0.6 to 1x10-6 m2/s for unconsolidated sediments. Over 10, 100, and 1000 yr, the calculation yields maximum of 18 m, 56 m, and 178 m, respectively, regardless of the magnitude of DT. In real situations, DT is usually small and may have short- (e.g., seasonal) or long-term fluctuations that swamp the signal associated with climate warming trends. Even over 103 yr, only gas hydrates close to the seafloor and initially within a few degrees of the thermodynamic stability boundary might experience dissociation in response to reasonable rates of warming. As discussed below, less than 5% of the gas hydrate inventory may meet these criteria.
Even when gas hydrate dissociates, several factors mitigate the impact of the liberated CH4 on the sediment-ocean-atmosphere system. In marine sediments, the released CH4 may dissolve in local pore waters, remain trapped as gas, or rise toward the seafloor as bubbles. Up to 90% or more of the CH4 that reaches the sulfate reduction zone (SRZ) in the near-seafloor sediments may be consumed by anaerobic CH4 oxidation (Hinrichs & Boetius 2002, Treude et al. 2003, Reeburgh 2007, Knittel & Boetius 2009). At the highest flux sites (seeps), the SRZ may vanish, allowing CH4 to be injected directly into the water column or, in some cases, partially consumed by aerobic microbes (Niemann et al. 2006).
Methane emitted at the seafloor only rarely survives the trip through the water column to reach the atmosphere. At seafloor depths greater than ~100 m, O2 and N2 dissolved in ocean water almost completely replace CH4 in rising bubbles (McGinnis et al. 2006). Within the water column, oxidation by aerobic microbes is an important sink for dissolved CH4 over some depth ranges and at some locations (e.g., Mau et al. 2007). These oxidizing microbial communities are remarkably responsive to environmental changes, including variations in CH4 concentrations. For example, rapid deepwater injection of large volumes of CH4 led to dramatically increased oxidation in the northern Gulf of Mexico in 2010 (Kessler et al. 2011, Yvon-Lewis et al. 2011). Water column CH4 oxidation mitigates the direct GHG impact of CH4 that is emitted at the seafloor, but it also depletes water column O2, acidifies ocean waters, and leads to the eventual release of the product CO2 to the atmosphere after residence times (Liro et al. 1993) of <50 years (water depths up to 500 m) to several hundred years (more profound water depths).
Global Warming and Gas Hydrate Type Locales
Methane hydrates occur in five geographic settings (or sectors) that must be individually evaluated to determine their susceptibility to warming climate (Figure 1). The percentages assigned to each sector below assume that 99% of global gas hydrate is within the deepwater marine realm (McIver 1981, Collett et al. 2009). Future refinements of the global ratio of marine to permafrost-associated gas hydrates will require adjustment of the assigned percentages. Owing to the orders of magnitude uncertainty in the estimated volume of CH4 trapped in global gas hydrate deposits, the percentages below have not been converted to Gt C.
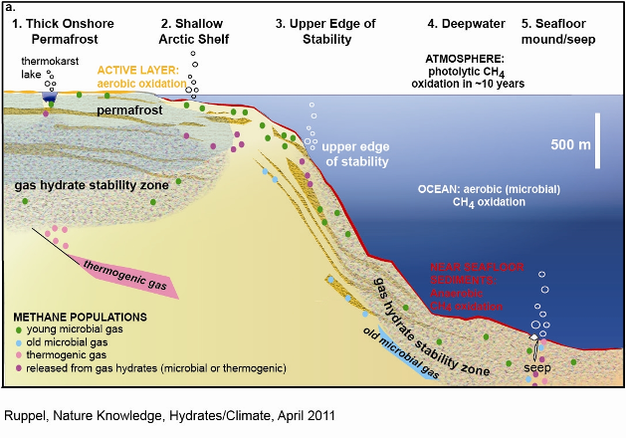
1. Thick (> 300 m) continuous permafrost onshore (<1%).
Deep gas hydrates beneath capping, permafrost-bearing sediments are stable over warm periods that endure more than 103 yr (e.g., Lachenbruch et al. 1994), even under scenarios of doubling atmospheric CO2 (Majorowicz et al. 2008). Only gas hydrates at the top of the GHSZ, nominally at ~225 m depth for pure CH4 hydrate within permafrost, might be vulnerable to dissociation due to atmospheric warming over 103 yr. Such shallow, intrapermafrost gas hydrate has been sampled in the North American Arctic (Collett et al. 2011, Dallimore & Collett 1995), but is not necessarily ubiquitous at high latitudes. Warming Arctic temperatures tracked in deep boreholes since the 1960s provide no evidence for climate perturbations reaching as deep as 200 m (Judge & Majorowicz 1992, Lachenbruch & Marshall 1986) in normal (e.g., not beneath lakes) continuous permafrost. Some researchers have argued that gas hydrates formed during previous periods of ice/water loading may persist today at subsurface depths as shallow as 20 m in areas of continuous permafrost (Chuvilin et al. 1998). Although their existence is controversial, such shallow gas hydrates would clearly be highly susceptible to dissociation in response to climate warming.
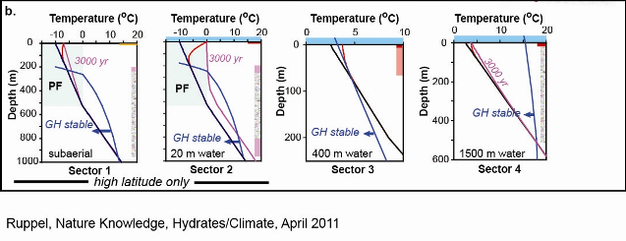
Simple numerical model: Adopting a pre-warming surface temperature of -10ºC and initial geotherms of 19ºC/km and 30ºC/km within and beneath permafrost, respectively, sustained surface warming over 100 yr with DT =3ºC at the surface does not raise temperatures enough to perturb the top of the GHSZ (Figure 1b). Warming over 3000 yr would lead to some thinning of permafrost and to dissociation of intrapermafrost gas hydrates in the shallowest part of the GHSZ.
Example: In the Mackenzie Delta, where permafrost is several hundreds of meters thick, Bowen et al. (2008) document seep gas composition similar to that of gas sequestered in nearby gas hydrates. There is no proof that active gas hydrate dissociation, as opposed to leakage of compositionally-similar free gas, supplies these seeps or other CH4 ebullition sites that occur throughout the Arctic region (e.g., Walter et al. 2007). Such sites should continue to be evaluated for evidence of a potential link to climate-driven gas hydrate dissociation.
2. Subsea permafrost on the circum-Arctic shelves (<0.25%?).
Sediments on shallow marine continental shelves that fringe the Arctic Ocean are often underlain by permafrost and associated gas hydrates that formed in Pleistocene time, when these regions were subaerial and exposed to much colder annual temperatures. Since the Late Pleistocene, marine inundation of these former coastal plains has led to large (up to 17ºC; Shakhova et al. 2010) temperature increases, partial thawing of subsea permafrost (Rachold et al. 2007), and inferred dissociation of gas hydrates (Semiletov et al. 2004). Increasing pressures (~1 MPa for 100 m of sea level rise since ~15 ka) would have only marginally offset the impact of warming temperatures on the GHSZ. Assuming that (a) 25% of northern-latitude continuous permafrost may have been flooded by Arctic Ocean transgressions since the Late Pleistocene, (b) some of this gas hydrate has dissociated over the past 10 kyr, and (c) less than 1% of the present-day global gas hydrate inventory is associated with permafrost implies that only a fraction of 1% of the global inventory occurs in areas of subsea permafrost. This estimate deserves considerable scrutiny in the coming years. Shakhova et al. (2010a) calculate that gas hydrates on the ESAS should sequester 20% of the carbon (375 Gt C) of the 1.8x103 Gt C within the conservative global gas hydrate inventory estimate (Boswell & Collett 2011).
Simple numerical model: Using the same initial conditions as for the terrestrial permafrost in Sector 1, a sustained temperature increase of DT =10ºC and an accompanying minor pressure increase are applied to mimic the impact of marine inundation of formerly subaerial permafrost to a water depth of 20 m (Figure 1b). After 100 yr, the temperature perturbation has propagated just to the top of gas hydrate stability. After 3000 yr, the permafrost has thawed, and gas hydrates located near the top and the base of the GHSZ are dissociating and releasing CH4.
Example: Shakhova et al. (2010) document CH4 supersaturation in shallow ESAS coastal waters above sediments containing degrading subsea permafrost and presumably dissociating gas hydrates. Studies are underway in similar settings on the Beaufort Sea inner shelf (e.g., Paull et al. 2011, Ruppel et al. 2010). A substantial fraction of CH4 that is emitted at the seafloor on Arctic shelves may reach the atmosphere since bubble dissolution and aerobic oxidation should be limited in such shallow (~5 to 50 m) waters. The challenge lies in proving that at least some of the elevated CH4 concentrations detected in these settings is attributable to dissociating gas hydrates rather than to other processes associated with CH4 generation and/or migration.
3. Deepwater marine hydrates at the feather edge of GHSZ (~3.5%).
The deepwater marine hydrate system thins to vanishing at shallow water depths (usually < 500 m) on the upper continental slopes. Because the entire GHSZ lies near the seafloor, upper continental slopes are the most susceptible places on Earth for wholesale gas hydrate dissociation driven by warming of impinging intermediate ocean waters. A maximum 3.5% of the global gas hydrate inventory might occur in these vulnerable settings assuming that (a) appropriate temperature-depth conditions for over the formation of a thin GHSZ occur over ~6x105 km2 of the upper continental slopes; (b) the GHSZ (3% saturation) is 40-m-thick; (c) the SRZ is missing owing to high seepage rates; and (d) warming climate does not necessarily raise water temperatures everywhere (e.g., Biastoch et al. 2011). As upper continental slope gas hydrate dissociates, the upper edge of the GHSZ moves downslope, priming more near-seafloor gas hydrate for dissociation. Dissolution of CH4 bubbles or oxidation of CH4 in the water column should prevent most of the CH4 that could be released from these gas hydrates from reaching the atmosphere immediately or in the form of CH4.
Simple Numerical Model: With an initial thermal gradient of 30ºC/km and original seafloor temperature of 2.5ºC, 100 yr of sustained warming (DT =1.25ºC) of the intermediate waters that impinge on the upper continental slope leads to complete dissociation of the gas hydrate zone, originally ~40 m thick (Figure 1b).
Example: At water depths of 150 to 400 m on the West Spitsbergen continental margin, widespread gas seepage may reflect gas hydrate dissociation caused by ~1ºC of ocean warming over the last 30 years (Westbrook et al. 2009). The connection between dissociating gas hydrates and the methane plumes has yet to be fully established, but observations there, in the Barents Sea (Lammers et al. 1995), and on the Canadian Beaufort Sea slope (Paull et al. 2011) provide compelling circumstantial evidence.
4. Deepwater gas hydrates (~95.5%).
These gas hydrates, which constitute most of the global inventory, generally have low susceptibility to warming climate over time scales shorter than a millennium. The gas hydrates closest to the edge of thermodynamic stability lie deep within the sedimentary section and close to the base of the GHSZ. Sustained bottom water temperature increases lasting many 103 yr would be required to initiate warming, no less dissociation. Even if CH4 is released from gas hydrate and is able to migrate toward the seafloor, some CH4 may be trapped in newly formed gas hydrate (e.g., Reagan & Moridis 2008) and much will be consumed in the SRZ.
Simple Numerical Model: Using the same initial conditions as for Sector 3 and assuming an increase in bottom water temperature of DT =1.25ºC, sustained warming over 100 and 3000 yr produces no dissociation of gas hydrate (Figure 1b).
5. Seafloor gas hydrate mounds (trace).
Conclusions
Catastrophic, widespread dissociation of methane gas hydrates will not be triggered by continued climate warming at contemporary rates (0.2ºC per decade; IPCC 2007) over timescales of a few hundred years. Most of Earth's gas hydrates occur at low saturations and in sediments at such great depths below the seafloor or onshore permafrost that they will barely be affected by warming over even 103 yr. Even when CH4 is liberated from gas hydrates, oxidative and physical processes may greatly reduce the amount that reaches the atmosphere as CH4. The CO2 produced by oxidation of CH4 released from dissociating gas hydrates will likely have a greater impact on the Earth system (e.g., on ocean chemistry and atmospheric CO2 concentrations; Archer et al. 2009) than will the CH4 that remains after passing through various sinks.
Contemporary and future gas hydrate degradation will occur primarily on the circum-Arctic Ocean continental shelves (Sector 2; Macdonald 1990, Lachenbruch et al. 1994, Maslin 2010), where subsea permafrost thawing and methane hydrate dissociation have been triggered by warming and inundation since Late Pleistocene time, and at the feather edge of the GHSZ on upper continental slopes (Sector 3), where the zone's full thickness can dissociate rapidly due to modest warming of intermediate waters. More CH4 may be sequestered in upper continental slope gas hydrates than in those associated with subsea permafrost; however, CH4 that reaches the seafloor from dissociating Arctic Ocean shelf gas hydrates is much more likely to enter the atmosphere rapidly and as CH4, not CO2. Proof is still lacking that gas hydrate dissociation currently contributes to seepage from upper continental slopes or to elevated seawater CH4 concentrations on circum-Arctic Ocean shelves. An even greater challenge for the future is determining the contribution of global gas hydrate dissociation to contemporary and future atmospheric CH4 concentrations.
Acknowledgements.
Glossary
Carbon isotopic excursion (CIE): Climate scientists use isotopic signatures recorded in ice cores, deep ocean sediments, thick carbonate sequences, and other types of samples to reconstruct Earth's climate history and the composition of the ocean/atmosphere. 13C isotopic signatures are commonly expressed as a δ13C value. Deviations from the baseline δ13C value are termed carbon isotopic excursions. Negative deviations in δ13C of even 1 part per mille (written as 1‰) are substantial for records constructed from benthic and planktonic foraminifera tests. Such deviations imply the emission of large amounts of isotopically-light carbon (strongly negative δ13C ratio) that likely originated through microbial methanogenesis. Large, climate-sensitive sources for isotopically-light methane carbon in the Earth system include wetlands and most natural gas hydrates.
Dissociation: The breakdown of gas hydrate occurs by dissociation to its constituent water and gas.
Gas hydrate: Gas hydrate is an ice-like solid that forms in sediments and remains stable at certain pressure-temperature conditions. Gas hydrates have a clathrate structure: water molecules form linked cages that enclose individual molecules of low molecular weight gas (e.g., CH4, CO2, hydrogen sulfide, ethane). Some water cages may be empty, rendering gas hydrates non-stoichiometric compounds and making it difficult to predict exactly how much gas will be released for dissociation of a given volume of gas hydrate. Gas hydrates in nature contain mostly methane as the trapped gas. This methane can originate from the microbial degradation of organic carbon or from deep burial and heating of organic matter, a so-called thermogenic process similar to that responsible for oil generation. Outside of hydrocarbon provinces, gas hydrate typically contains almost exclusively microbial methane.
Hyperthermals: Periods characterized by extremely warm climate conditions.
Saturation: The percentage of sediment pore space occupied by gas hydrate.
Sulfate reduction zone (SRZ): A zone within the marine sedimentary section starting at the sediment-water interface and extending downward to the depth at which sulfate is depleted as a result of the microbially-mediated oxidation of methane and other organic compounds. The thickness of the SRZ varies from centimeters to tens of meters and is inversely related to the upward flux of methane toward the seafloor and the organic matter content of the sediments. Most of the methane that enters the SRZ from below is consumed before it reaches the seafloor.
References and Recommended Reading
Archer, D. et al. Ocean methane hydrates as a slow tipping point in the global carbon cycle. Proceedings of the National Academy of Sciences of the United States of America 106, 20956-20601 (2009). doi:10.1073/pnas.0800885105
Biastoch, A. et al. Rising Arctic Ocean temperatures cause gas hydrate destabilization and ocean acidification. Geophysical Research Letters 38, L08602 (2011). doi:10.1029/2011GL047222
Bock, M. et al. Hydrogen isotopes preclude marine hydrate CH4 emissions at the onset of Dansgaard-Oeschger events. Science 328, 1686-1689 (2010).
Bohannon, J. Weighing the climate risks of an untapped fossil fuel. Science 319, 1753 (2008).
Boswell, R. & Collett, T. S. Current perspectives on gas hydrate resources. Energy and Environmental Science 4, 1206-1215 (2011).
Bowen, R. G. et al. "Geomorphology and gas release from pockmark features in the Mackenzie Delta, Northwest Territories, Canada," in Proceedings of the 9th International Conference on Permafrost, eds. D. L. Kane & K. M. Hinkel (Fairbanks, AK: Institute of Northern Engineering, 2008) 171-176.
Chuvilin, E. et al. Gas hydrates in the permafrost of Bovanenkovo gas field, Yamal Peninsula, West Siberia. Polarforschung 68, 215-219 (2000).
Collett, T. S. et al. Permafrost associated natural gas hydrate occurrences on the Alaskan North Slope. Marine and Petroleum Geology 28, 279-294 (2011). doi:10.1016/j.marpetgeo.2009.12.001
Collett, T. S. et al. "Natural gas hydrates: A review," in Natural Gas Hydrates-Energy Resource Potential and Associated Geologic Hazards, eds. T. Collett et al. (Tulsa, OK: American Association of Petroleum Geologists, 2009) 146-219.
Dallimore, S. R. & Collett, T. S. Intrapermafrost gas hydrates from a deep core hole in the Mackenzie Delta, Northwest Territories, Canada. Geology 23, 527-530 (1995).
Dickens, G. R. et al. Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the Paleocene. Paleoceanography 10, 965-971 (1995).
Dickens, G. R., Down the rabbit hole: Toward appropriate discussion of methane release from gas hydrate systems during the Paleocene-Eocene thermal maximum and other past hyperthermal events. Climate of the Past 7, 831-846 (2011). doi:10.5194/cp-7-831-2011
Fung, I. et al. Three-dimensional model synthesis of the global methane cycle. Journal of Geophysical Research 96, 13033-13065 (1991).
Harvey, L. D. D. & Huang, Z. Evaluation of potential impact of methane clathrate destabilization on future global warming. Journal of Geophysical Research 100, 2905-2926 (1995).
Hesselbo, S. P. et al. Massive dissociation of gas hydrate during a Jurassic anoxic event. Nature 406, 392-395 (2000).
Hinrichs, K-U. & Boetius, A. "The anaerobic oxidation of methane: New insights in microbial ecology and biochemistry," in Ocean Margin Systems, eds. G. Wefer et al. (Berlin, Germany: Springer-Verlag, 2002) 457-477.
Hu, L. et al. Methane fluxes to the atmosphere from deepwater hydrocarbon seeps in the northern Gulf of Mexico. Journal of Geophysical Research in press (2011). doi:10.1029/2011JC007208
IPCC (Intergovernmental Panel on Climate Change). Climate Change 2001: The Scientific Basis. New York, NY: Cambridge University Press, 2001.
IPCC (Intergovernmental Panel on Climate Change). Climate Change 2007: The Physical Basis.New York, NY: Cambridge University Press, 2007.
Jiang, G. et al. Stable isotope evidence for methane seeps in Neoproterozoic postglacial cap carbonates. Nature 426, 822-826 (2003).
Judge, A. S. & Majorowicz, J. A. Geothermal conditions for gas hydrate stability in the Beaufort-Mackenzie area: The global change aspect. Palaeogeography, Palaeoclimatology, Palaeoecology 98, 251-263 (1992).
Kennett, J. P. et al. Methane Hydrates in Quaternary Climate Change-The Clathrate Gun Hypothesis. Washington, DC: American Geophysical Union, 2003.
Kessler, J. D. et al. A persistent oxygen anomaly reveals the fate of spilled methane in the deep Gulf of Mexico. Science 331, 312-315 (2011). doi:10.1126/science.1199697
Krey, V. et al. Gas hydrates: Entrance to a methane age or climate threat? Environmental Research Letters 4, 034007 (2009). doi:10.1088/1748-9326/4/3/034007
Lachenbruch, A. H. Permafrost, the Active Layer, and Changing Climate. United States Geological Survey, Open File Report 94-694 (1994).
Lachenbruch, A. H. & Marshall, B. V. Changing climate: Geothermal evidence from permafrost in the Alaskan Arctic. Science 234, 689-696 (1986).
Lammers, S. et al. A large methane plume east of Bear Island (Barents Sea): Implications for the marine methane cycle. Geologische Rundschau 84, 59-66 (1995).
Lelieveld, J. et al. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus 50B, 128-150 (1998).
Liro, C. R. et al. Modeling the release of CO2 in the deep ocean. Energy Conversion and Management 33, 667-674 (1992).
Macdonald, G. Role of methane clathrates in past and future climate. Climatic Change 16, 247-281 (1990).
Macdonald, I. R. et al. Thermal and visual time-series at a seafloor gas hydrate deposit on the Gulf of Mexico slope. Earth and Planetary Science Letters 233, 45-59 (2005). doi:10.1016/j.epsl.2005.02.002
Macdonald, I. R. et al. Gas hydrate that breaches the seafloor on the continental slope of the Gulf of Mexico. Geology 22, 699-702 (1994).
Majorowicz, J. A. et al. "Onset and stability of gas hydrates under permafrost in an environment of surface climatic change-past and future," in Proceedings of the 6th International Conference on Gas Hydrates. Vancouver, BC: ICGH, 2008.
Mascarelli, A. L. A sleeping giant? Nature Reports Climate Change 3, 46-49 (2009).
Maslin, M. et al. Linking continental-slope failures and climate change: Testing the clathrate gun hypothesis. Geology 32, 53-56 (2004). doi:10.1130/G20114.1
Maslin, M. et al. Gas hydrates: Past and future geohazard. Philosophical Transations of the Royal Society A: Mathematical, Physical & Engineering Sciences 368, 2369-2393 (2010). doi:10.1098/rsta.2010.0065
Mau, S. et al. Dissolved methane distributions and air-sea flux in the plume of a massive seep field, Coal Oil Point, California. Geophysical Research Letters 34, L22603 (2007). doi:10.1029/2007GL031344
McGinnis, D. F. et al. Fate of rising methane bubbles in stratified waters: How much methane reaches the atmosphere? Journal of Geophysical Research 111, C09007 (2006). doi:10.1029/2005JC003183
McIver, R. D. "Gas hydrates," in Long-Term Energy Resources, eds. R. F. Meyer & J. C. Olson (Boston, MA: Pitman, 1981) 713-726.
Niemann, H. et al. Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature 443, 854-858 (2006). doi:10.1038/nature05227
Paull, C. et al. "Tracking the decomposition of submarine permafrost and gas hydrate under the shelf and slope of the Beaufort Sea," in Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, Scotland: ICGH, 2011.
Petrenko, V. et al. 14CH4 measurements in Greenland ice: Investigating last glacial termination CH4 sources. Science 324, 506-508 (2009). doi:10.1126/science.1168909
Rachold, V. et al. Near-shore arctic subsea permafrost in transition. Eos, Transactions of the American Geophysical Union 88, 149-56 (2007).
Reagan, M. T. & Moridis, G. J. Dynamic response of oceanic hydrate deposits to ocean temperature change. Journal of Geophysical Research 113, C12023 (2008). doi:10.1029/2008JC004938
Reeburgh, W. S. Oceanic methane biogeochemistry. Chemical Reviews 107, 486-513 (2007).
Renssen, H. et al. Modeling the climate response to a massive methane release from gas hydrates. Paleoceanography 19, PA2010 (2004). doi:10.1029/2003PA000968
Röhl, U. et al. On the duration of the Paleocene-Eocene Thermal Maximum. Geochemistry, Geophysics, Geosystems 8, Q12002 (2007). doi:10.1029/2007GC001784
Ruppel, C. et al. Degradation of subsea permafrost and associated gas hydrates offshore of Alaska in response to climate change. Sound Waves 128, 1-3 (2010). http://soundwaves.usgs.gov/2010/11
Schmidt, G. A. & Shindell, D. T. Atmospheric composition, radiative forcing, and climate change as consequence of a massive methane release from gas hydrates. Paleoceanography 18, 1004 (2003). doi:10.1029/2002PA000757
Semiletov, I. et al. Methane climate forcing and methane observations in the Siberian Arctic Land-Shelf system. World Resource Review 16, 503-543 (2004).
Shakhova, N. et al. Geochemical and geophysical evidence of methane release over the East Siberian Arctic Shelf. Journal of Geophysical Research 115, C08007 (2010a) doi:10.1029/2009JC005602
Shakhova, N. et al. Extensive methane venting to the atmosphere from sediments of the East Siberian Arctic Shelf. Science 327, 1246-1250 (2010b). doi:10.1126/science.1182221
Solomon, E. A. et al. Considerable methane fluxes to the atmosphere from hydrocarbon seeps in the Gulf of Mexico. Nature Geoscience 2, 561-565 (2009). doi:10.1038/NGE0574
Sowers, T. Late Quaternary atmospheric CH4 isotope record suggests marine clathrates are stable. Science 311, 838 (2006). doi:10.1126/science.1121235
Suess E. et al. "Sea floor methane hydrates at Hydrate Ridge, Cascadia Margin," in Natural Gas Hydrates-Occurrence, Distribution and Detection, eds. W. P. Dillon & C. K. Paull (Washington, DC: American Geophysical Union, 2001) 87-98.
Treude, T. et al. Anaerobic oxidation of methane at Hydrate Ridge (OR). Geochimica et Cosmochimica Acta 67, A491-A491 (2003).
Tryon, M. D. et al. Fluid and chemical flux in and out of sediments posting methane hydrate deposits on Hydrate Ridge, OR, II: Hydrological processes. Earth and Planetary Science Letters 201, 541-557 (2002).
Walter, K. M. et al. Methane bubbling from northern lakes: Present and future contributions to the global methane budget. Philosophical Transactions of the Royal Society A: Mathematical, Physical & Engineering Sciences 365, 1657-1676 (2007).
Wang, J. S. et al. A 3-D model analysis of the slowdown and interannual variability in the methane growth rate from 1988 to 1997. Global Biogeochemical Cycles 18, GB3011 (2004).
Westbrook, G. K. et al. Escape of methane gas from the seabed along the West Spitsbergen continental margin. Geophysical Research Letters 36, L15608 (2009) doi:10.1029/2009GL039191
Yvon-Lewis, S. A. et al. Methane flux to the atmosphere from the Deepwater Horizon oil disaster. Geophysical Research Letters 38, L01602 (2011). doi:10.1029/2010GL045928
Zachos, J. et al. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686-693 (2001).
Zachos, J. et al. Rapid acidification of the ocean during the Paleocene-Eocene thermal maximum. Science 308, 1611-1615 (2005). doi:10.1126/science.1109004






























