« Prev Next »

People have pondered animal behavior since the beginnings of human existence (Frison 1998). Insights into animal behavior allowed our ancestors to outsmart prey during a hunt or befriend another animal, the latter eventually leading to domestication of animals. Knowledge of animal mating systems helped human societies grow, because propagation of domesticated stocks ensured that there were dependable supplies of food (Zeder 2008).
When observing wild or domesticated animals today, we are often fascinated by their persistence in accomplishing a given task, such as when magpies adjust individual sticks to form a nest or when a pet seeks out its favorite toy (Figure 1). And, we continue to ask basic questions about animal behavior: Do animals have motivations and preferences? Do they make conscious choices? When given alternative partners or various foods to choose among, do animals make adaptive choices that are good for them? These and similar questions are addressed in controlled experimental designs that measure and test connections between an observed behavior and its hypothesized affectors.

What are Motivations, Preferences and Choices, and Why Do Animals Have Them?
Motivation, the desire or willingness to engage in a behavior, can be positive or negative and vary in strength. Motivation is positive for tasks and outcomes that animals wish for and negative for undesirable activities. Positive and negative motivations are inferred from behavior, for example, from approach-behavior vs. avoidance, respectively (Kirkden & Pajor 2006).
Motivational strength varies between individuals. Some components of this variation can be ascribed to constant individual factors like species-specific characteristics or gender, while others are explained by changing individual or environmental factors like age, experience, time of day, weather, and resource predictability. In other words, a motivation is generally influenced by many factors that can be intrinsic (e.g., genetic or physiological) or extrinsic (i.e., in the animal’s environment). To illustrate, the motivation to drink (thirst) can be increased by hormones responsible for controlling the body’s water balance, but is also enhanced by the sight of water.
Preferences are based on an ability to evaluate sets of simultaneously available alternatives that satisfy the same motivation and to aspire toward the one opportunity that is most desirable. A preference may be specific to the individual (such as preferring potato chips over nuts), and refers to the difference in motivational strength to get the one resource over the other, or others. Ultimately, animal preferences are inferred from choice behavior. Choice behavior refers to what an animal actually does — the consequences of its preferences and its final decisions.
In summary, before animals make choices they go through a decision-making process guided by their motivations and preferences. Presumably, this strategy is adaptive, insofar as being advantageous to the animal and favored by evolution via natural selection: Individuals that choose beneficial options, like eating the more nutritious food or resting at the safer nesting site, are also more likely to have offspring that are successful in reproduction and survival (Real 1991).
How Can We Assess What Animals Prefer and the Strength of Their Preferences?
It is feasible to measure animal choice behavior in the wild and make inference about what animals prefer. Jane Goodall, for example, used ad libitum behavior sampling to reveal details about the preferred placement of chimpanzees’ sleeping-nests in Tanzania. She found that nests typically were built in rather inaccessible places, presumably as a predator avoidance strategy (Goodall 1962). Thus, behavioral data from field studies can provide useful information about animal choice behavior, but the results can also be challenging to interpret because many factors that influence behavior are outside the researcher’s control.
Many scientists examine animal behavior in standardized test situations in the laboratory. In this setting, behavioral affectors are more easily monitored and controlled for than in the wild. For reliable control over experimental variables and optimal power to test behavioral affectors, laboratory studies are usually performed with captive-born or domesticated animals that are familiar with semi-natural or artificial housing situations. In the laboratory, an animal’s preferences are tested by allowing it to choose between two or more defined options. Next, when the animal’s choice behavior reveals the option that is preferred or avoided in the experiment, the scientist can move on to ask how strong its behavioral preference is. Such tests are termed motivation tests.
In laboratory testing of factors that can influence behavior, treatment animals, which are exposed to the factor or factors of interest, are contrasted to reference observations. In some experiments, the reference observations are made initially to determine the baseline behavior of each animal. After treatment, the animal is compared to its own baseline as a reference or internal control. In other experimental designs, separate subsets of individuals form a reference or control group that parallels the treatment in all aspects other than the factor(s) of interest. Subsequent comparisons of the control and treatments groups allow scientists to conclude about the influences on behavior, with fewer disturbances from intrinsic and extrinsic elements that can confound interpretation.
Case Study 1: Social Preference and Motivational Strength
Among dogs, it is important for young animals to interact with others for play and social learning, and to feel safe. The same is true for other canids including captive silver foxes, known in the wild as the red fox (Vulpes vulpes). At two months of age, fox pups prefer social contact and respond towards both familiar and unfamiliar pups in a playful manner. However, as the foxes age from pups to seven-month-old juveniles, they spend less time with a social companion and their behavior towards strangers changes from playful to aggressive (Akre et al. 2009).
How can we measure whether juvenile foxes are motivated for social contact?
A method for testing an animal’s strength of motivation is to ask it to "pay" an entrance fee for admission to a resource and then measure the maximum "price" it is willing to pay. But what sort of currency can be used with animals like foxes? The currency is typically a learned behavior, like pushing a heavy door or pulling a loop string, a so-called operant response. Also, when using operant methodology to test whether social contact is important; the price foxes pay for access to a companion must be compared with access to a baseline resource of known importance to them. One such resource is food, since animals are motivated to satisfy their hunger (Jackson et al. 1999). The foxes’ valuation of food thereby becomes the internal reference-behavior of this study.
This system (Figure 2) was used to test the strength of social motivations in six juvenile female silver foxes that had been trained to pull a loop to open a door. When a fox delivered a required number of pulls, the door opened into a chamber. The foxes’ motivations were examined in two separate trials: During one trial, the foxes worked for food placed inside the chamber to establish their individual reference value. During the other trial, the foxes could socialize with another female when they visited the chamber. The companion could freely enter or leave, which insured that being together was voluntary.
In each trial, the experimenters raised the entrance fee daily by requiring more pulls before the door would open, until each animal had paid its maximum price and ended its visit to the chamber.
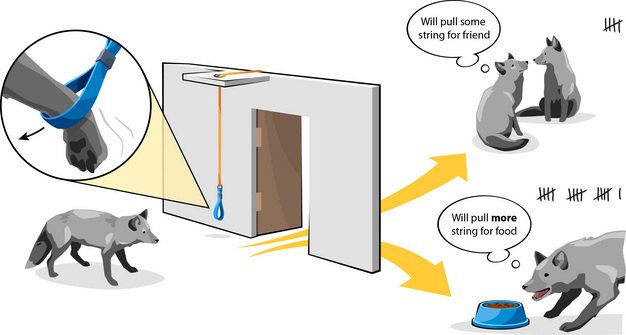
The researchers could now work out the average price the juvenile foxes were willing to pay for each resource. Food was the most desired commodity with an average of 1632 pulls between the six females (min 224, max 2368). However, the price paid for social contact averaged as much as 512 pulls (min 128, max 992), which represented 38% of the value that the foxes placed on food when hungry. Thus, the researchers concluded that female silver foxes do desire social contact as juveniles (Hovland et al. 2008).
Such operant experiments, relying on simple behavioral responses that allow animals to show us what they want, can give valuable insight into resources that are important for animal well-being.
Case Study 2: Food Preference and the Physiology of Choice Behavior
Among honey bees (Apis mellifera), individuals participate in social food-gathering outside the colony. Some bees prefer protein-containing pollen from flowering plants, while others seek out nectar — a rich source of carbohydrates. Bees with different foraging preferences also show different expression-levels of insulin-sensing genes in their abdominal fat (Amdam & Page 2010). Insulin sensing is active in muscles, liver and fat during uptake of sugar (glucose) from the blood, but insulin signals can also act remotely on the brain to regulate eating behavior (Vettor et al. 2002).
How can we test whether insulin-sensing genes in the bees’ abdominal fat can act remotely on the brain, to affect behavioral preferences toward pollen and nectar collection?
Genetic effects on behavior can be studied in laboratory animals where the genes of interest are "knocked down", so their expression is disrupted. Similar gene knockdown can be achieved in the abdominal fat cells of honey bees with a technique called RNA interference. Using RNA interference, scientists compared 150 knockdown honey bees toward a reference group with a similar number of control bees. The controls went through a matching technical and biochemical handling-procedure that did not cause gene knockdown. The knockdown treatment targeted IRS (the Insulin Receptor Substrate); a gene that is central to insulin sensing. The knockdown and control bees were tagged, transferred to honey bee colonies, and carefully observed. Tagged bees were captured when returning to their host colonies from foraging flights, and the researchers measured their gustatory (taste) response to sugar as well as how much pollen and nectar the bees collected (for more details, see Wang et al. 2010).
The scientists found that the treatment and control bees responded equally to sugar, but the IRS knockdown bees collected about 30% less nectar and proportionally more pollen than the controls. The study concluded that an insulin-sensing gene in the abdominal fat of honey bees could influence the insects’ foraging choice-behavior without altering the bees’ taste for sugar (Wang et al. 2010).
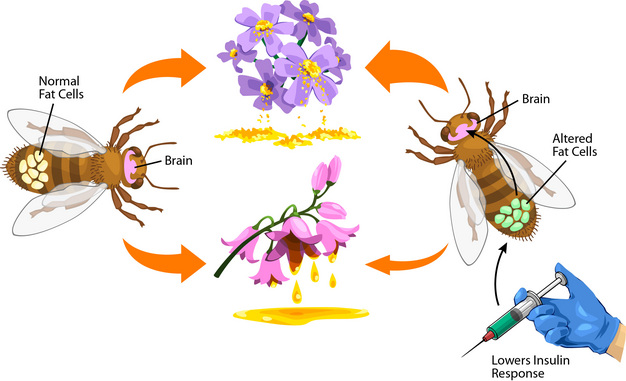
Such basic experiments on how physiology can bias individuals toward different food cravings can aid our progress in understanding food-related behavior and how it influences human health.
Glossary
Adaptive behavior – Any behavior that enables an animal to adjust to a specific situation or environment, and that supports the animal’s long-term survival and reproduction.
Ad libitum behavior sampling – To record all instances of behavior that seem appropriate in an animal or in groups of animals. Requires no specific rules for how the behavior is recorded or which animals are recorded.
Decision-making – To evaluate the perceived stimulus and select an appropriate behavioral response. The process of decision-making can be simple (e.g., rely on sensory filters), or involve complex cognitive processes like problem-solving.
Experimental controls – Subjects that do not receive experimental treatment but are similar to treated subjects in all other respects.
Experimental paradigm – Established methods that allow experiments to be conducted by specific standards. Typically rely on theory that guides methods-development and use.
Operant response – A learned behavior established based on the consequences of its performance.
RNA interference – Enzymatic cleavage of an mRNA based on its recognition by the RNA-induced silencing complex (RISC). Recognition uses a short template RNA in RISC that is complimentary to a segment of the target mRNA sequence. RISC-mediate cleavage of mRNA can silence gene expression by reducing the amount of mRNA available for protein synthesis.
References and Recommended Reading
Amdam, G. V. & Page, R. E. The developmental genetics and physiology of honeybee societies. Animal Behaviour 79, 973–980 (2010).
Frison, G. C. Paleoindian large mammal hunters on the plains of North America. Proceedings of the National Academy of Sciences of the United States of America 95, 14576–14583 (1998).
Goodall, J. Nest building behavior in free ranging chimpanzee. Annals of the New York Academy of Sciences 102, 455–467 (1962).
Hovland, A. L. et al. The nature and strength of social motivations in young farmed silver fox vixens. Applied Animal Behaviour Science 111, 357–372 (2008).
Jackson, R. E., Waran, N. K., & Cockram, M. S. Methods for measuring feeding motivation in sheep. Animal Welfare 8, 53–63 (1999).
Kirkden, R. D. & Pajor, E. A. Using preference, motivation and aversion test to ask scientific questions about animals’ feelings. Applied Animal Behaviour Science 100, 29–47 (2006).
Real, L. A. Animal choice behavior and the evolution of cognitive architecture. Science 253, 980–986 (1991).
Vettor, R. et al. Neuroendocrine regulation of eating behavior. Journal of Endocrinological Investigation 25, 836–854 (2002).
Wang, Y. et al. Down-regulation of honey bee IRS gene biases behavior toward food rich in protein. PLoS Genetics 6:e1000896 (2010).
Zeder M. A. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proceedings of the National Academy of Sciences of the United States of America 105, 11597–11604 (2008).






























