« Prev Next »

Reproductive strategies represent a set of behavioral, morphological, and physiological adaptations that facilitate access to potential mates, improve the chances of mating and fertilization, and enhance infant survival. Reproductive strategies of primate males and females differ because of fundamental sex differences in potential reproductive rates characteristic of all mammals (Trivers 1972, Clutton-Brock & Parker 1992). Because of evolutionary constraints imposed by internal gestation and subsequent lactation, primate females bear the lion's share of parental investment, and their reproductive success is therefore limited by the quality of parental care. Female primates, therefore, have an interest in soliciting paternal care, which is only found in a minority of species, however (reviewed in Muller & Emery Thompson 2012). Males, in contrast, minimally contribute one ejaculate for successful reproduction, so that they can fertilize many more females while their mates are still gestating or lactating. Male mating success is therefore primarily limited by the number of fertile females to which they have access. The resulting difference in fitness-limiting factors necessitates a separate consideration of female and male reproductive strategies. Although the focus here is on male strategies, male reproductive success is crucially dependent on female choice and cooperation (Kappeler 2012) because sexual coercion of females into mating is only rarely an option for male primates (Smuts & Smuts 1993, Muller & Wrangham 2009, Knott et al. 2010).
Measurements of Reproductive Performance
The Ideal Male Reproductive Strategy
The optimal reproductive strategy of a hypothetical male primate is characterized by rapid sexual maturation, followed by life-long exclusive access to an unlimited number of fertile females willing to mate, and offspring survival should be independent of paternal care. In reality, however, males take time to grow and mature, they compete with rivals for exclusive mating access and more often for priority of access to receptive females, they are dependent on female choice and cooperation to achieve successful copulation, they suffer costs of mating effort (Hoffman et al. 2008, Kraus et al. 2008), and they may improve offspring survival and wellbeing through paternal care (Breuer et al. 2010). Appreciation of these constraints on an ideal strategy helps explain the diversity of existing outcomes because males face multiple strategic trade-offs with variable outcomes within and between species.
Variation in Male Reproductive Strategies
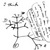
The extent of variation in male reproductive strategies between, but also within species can be illustrated by comparing four well-studied group-living species. In mountain gorillas (Gorilla beringei), about 40% of groups contain a second adult male besides the dominant silverback, and males are about twice the size of females. Silverbacks have an 85% probability of siring all offspring in a group during their tenure, and no infants are sired by extra-group males (Bradley et al. 2005). Despite a smaller number of adult females, virtually all groups of Verreaux's sifakas (Propithecus verreauxi) contain at least one other adult male, and males are slightly smaller than adult females. Dominant male sired 91% of their group's offspring in one population (Kappeler & Schäffler 2008), whereas 42% of all infants were attributed to an extra-group father in another population (Lawler 2007). In chimpanzees (Pan troglodytes), 7–12 adult males that are about 25% bigger than females, establish a linear dominance hierarchy, but the alpha male in one community fathered only about 30% of the infants (Wroblewski et al. 2010). Extra-group paternity in chimpanzees is rare (7%: Vigilant et al. 2001) or absent altogether. Finally, dominant male savannah baboons (Papio cynocephalus), who are about twice the size of females (Figure 1), and who compete with about the same number of rivals as chimpanzees, sire on average 34% of offspring (Alberts et al. 2006). Only 52% of male savannah baboons ever sired an infant, and the number of infants varied among these males between 1 and 16, but there was no extra-group paternity.

Thus, even without considering solitary and pair-living species, there is great variation in male reproductive skew (the partitioning of fertilizations among males) among and within species that requires explanation. One line of primatological research has traditionally focused on explaining interspecific differences by comparing aspects of the mating system and sexually-selected traits (Alberts 2012). For instance, the species in the above example differ in the number of adult males per group, the socionomic sex ratio (the proportion of males and females ready to mate at a given time) and the degree of sexual size dimorphism (male body size expressed as a proportion of female size). They also vary in the presence of female sexual swellings (anogenital skin of females gradually swells during the estrous cycle, usually reaching a maximum around ovulation; found in Pan and Papio in the example above; Figure 2), mating seasonality (only in Propithecus), and relative testes size (much smaller than expected in Gorilla and Propithecus). A complementary line of research has focused on the rules that determine patterns of reproductive skew. Theoretical models of reproductive skew focusing either on transaction (division of reproduction is the outcome of reproductive transactions between dominant and subordinate) or compromise (reproductive skew is the outcome of a struggle over reproduction between dominant and subordinate) cannot be used to explain the division of reproduction among male primates because primates violate their assumptions (Kutsukake & Nunn 2007, Port & Kappeler 2010). However, Priority-of-access models (a positive relation between rank and reproductive success) that incorporate queuing within groups and consider the options for males in neighboring groups as well (Port et al. 2010) promise to provide comprehensive explanations for patterns of reproductive skew and social organization.

Determinants and Mechanisms of Male Reproductive Strategies
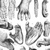
Whenever males cannot monopolize groups of females, they compete with a combination of mechanisms to enhance their individual probabilities of mating and fertilization. In most species, males establish dominance relations based on age, strength and dispersal status to mitigate the potential costs of direct aggression in the mating context. In the majority of cases, access to receptive females is rank-dependent, with alpha males enjoying the highest reproductive success (Altmann et al. 1996, Alberts et al. 2003). Mate guarding (a male prevents a female from mating with other males by maintaining close proximity during her receptive period; Figure 3) of estrous females is a widespread behavioral mechanisms used by males to implement their advantage over rivals. Male-male competition can also have physiological correlates, resulting in differences in stress and sex hormones between high- and low-ranking males (van Belle et al. 2009, Setchell et al. 2010). Subordinate males are therefore often in a position where they can only make the best of a bad job, for example by mating surreptitiously (Gibson 2010), by forming coalitions against higher-ranking males (Schülke et al. 2010), or by forming temporary friendships with females that confer mating privileges (Palombit et al. 1997). Dispersal to another group with better reproductive prospects provides another option for males that are unable to challenge the local top-ranking male(s) (Alberts & Altmann 1995).

Whenever males fail to monopolize matings with estrous females, competition for fertilization continues after copulation. Sperm competition (competition for fertilization among the sperm of two or more males) has resulted in a number of behavioral, physiological and anatomical adaptations that are exclusive to, or much more pronounced in, promiscuous species. Post-copulatory mate guarding may provide a male's own sperm a head start in the race for fertilization. There is also strong selection on males subject to sperm competition to produce more and larger ejaculates (which is facilitated by larger testes) and faster and more enduring spermatozoa (Anderson & Dixson 2002), leading to predictable species differences in these traits (Soulsbury 2010). Causes and consequences of intraspecific variation in these traits remain poorly studied (Bercovitch 1997). Even after fertilization, males of some species may be able to induce abortions, thereby negating the reproductive success of rivals (Beehner & Bergman 2008). More commonly, primate males kill dependent offspring they have not sired, thereby accelerating a mating opportunity with the respective mother (van Schaik 2000).
Because reproductive success is the ultimate measure of selection, behavioral tactics and other traits that confer an advantage either before or after copulation are under strong positive selection. These effects not only affect male assertiveness and aggression over evolutionary time, but also optimal schedules of growth, maturation and dispersal, as well as sexual dimorphism in morphological traits related to male competition (such as body and canine size or status-dependent ornaments) (Plavcan 2004). As a result of their smaller parental investment, males are also less concerned about the potential deleterious consequences of inbreeding than females. They do not allocate their mating effort indiscriminately, however. There is evidence that males may target their mating effort towards particular females that promise higher fertility, especially when the potential costs of male-male competition are high or when female reproductive synchrony breaks male monopolization potential (Alberts et al. 2006, Emery Thompson & Wrangham 2008). Male mate choice therefore constitutes a particularly promising topic for future research on male mating strategies. Finally, in a few primate species, males use aggression or the threat of aggression to control female sexuality (Muller & Wrangham 2009). Such sexual coercion can be direct (i.e., males use violence to overcome female resistance to mating) or indirect (i.e., they employ threats or aggression to decrease the chance that a female will mate with other males).
References and Recommended Reading
Alberts, S. C. "Magnitude and sources of variation in male reproductive performance," in Evolution of Primate Societies, eds. J. C. Mitani et al. (Chicago, IL: University of Chicago Press, 2012) 412-431.
Alberts, S. C. & Altmann, J. Balancing costs and opportunities: Dispersal in male baboons. The American Naturalist 145, 279-306 (1995).
Alberts, S. C. et al. Queuing and queue-jumping: Long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Animal Behaviour 65, 821-840 (2003).
Alberts, S. C. et al. Sexual selection in wild baboons: From mating opportunities to paternity success. Animal Behaviour 72, 1177-1196 (2006).
Altmann, J. et al. Behavior predicts genetic structure in a wild primate group. Proceedings of the National Academy of Sciences of the United States of America 93, 5797-5801 (1996).
Andelman, S. J. "Ecological and social determinants of cercopithecine mating patterns," in Ecological Aspects of Social Evolution: Birds and Mammals, eds. D. I. Rubenstein & R. W. Wrangham (Princeton, NJ: Princeton University Press, 1986) 201-216.
Anderson, M. J. & Dixson, A. F. Sperm competition: Motility and the midpiece in primates. Nature 416, 496 (2002).
Beehner, J. C. & Bergman, T. J. Infant mortality following male takeovers in wild geladas. American Journal of Primatology 70, 1152-1159 (2008).
Bradley, B. J. et al. Mountain gorilla tug-of-war: Silverbacks have limited control over reproduction in multimale groups. Proceedings of the National Academy of Sciences of the United States of America 102, 9418-9423 (2005).
Breuer, T. et al. Variance in the male reproductive success of western gorillas: Acquiring females is just the beginning. Behavioral Ecology and Sociobiology 64, 515-528 (2010).
Clutton-Brock, T. H. & Parker, G. A. Potential reproductive rates and the operation of sexual selection. Quarterly Review of Biology 67, 437-456 (1992).
Cords, M. "When are there influxes in blue monkey groups?" in The Guenons: Diversity and Adaptation in African Monkeys, ed. R. H. Tuttle (New York, NY: Springer, 2004) 189-201.
Eberle, M. & Kappeler, P. M. Sex in the dark: Determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behavioral Ecology and Sociobiology 57, 77-90 (2004).
Emery Thompson, M. & Wrangham, R. W. Male mating interest varies with female fecundity in Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park. International Journal of Primatology 29, 885-905 (2008).
Emlen, S. T. & Oring, L. W. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215-223 (1977).
Gibson, K. N. Male mating tactics in spider monkeys: Sneaking to compete. American Journal of Primatology 72, 794-804 (2010).
Hoffman, C. et al. Sex differences in survival costs of reproduction in a promiscuous primate. Behavioral Ecology and Sociobiology 62, 1711-1718 (2008).
Kappeler, P. M. "Mate choice," in Evolution of Primate Societies, eds. J. C. Mitani et al. (Chicago, IL: University of Chicago Press, 2012) 367-386.
Kappeler, P. M. & Schäffler, L. The lemur syndrome unresolved: Extreme male reproductive skew in sifakas (Propithecus verreauxi), a sexually monomorphic primate with female dominance. Behavioral Ecology and Sociobiology 62, 1007-1015 (2008).
Kappeler, P. M. et al. Even adult sex ratios in lemurs: Potential costs and benefits of subordinate males in Verreaux's sifaka (Propithecus verreauxi) in the Kirindy Forest CFPF, Madagascar. American Journal of Physical Anthropology 140, 487-497 (2009).
Knott, C. D. et al. Female reproductive strategies in orangutans, evidence for female choice and counterstrategies to infanticide in a species with frequent sexual coercion. Proceedings of the Royal Society B: Biological Sciences 277, 105-113 (2010).
Kraus, C. et al. The costs of risky male behavior: Sex differences in seasonal survival in a small sexually monomorphic primate. Proceedings of the Royal Society B: Biological Sciences 275, 1635-1644 (2008).
Kutsukake, N. & Nunn, C. L. Comparative tests of reproductive skew in male primates: The roles of demographic factors and incomplete control. Behavioral Ecology and Sociobiology 60, 695-706 (2006).
Lawler, R. Fitness and extra-group reproduction in male Verreaux's sifaka: An analysis of reproductive success from 1989-1999. American Journal of Physical Anthropology 132, 267-277 (2007).
Mitani, J. C. et al. Number of males in primate groups: Comparative tests of competing hypotheses. American Journal of Primatology 38, 315-332 (1996).
Muller, M. N. & Emery Thompson, M. "Mating, parenting and male reproductive strategies," in Evolution of Primate Societies, eds. J. C. Mitani et al. (Chicago, IL: University of Chicago Press, 2012) 387-411.
Muller M. N. & Wrangham, R. W. Sexual Coercion in Primates and Humans: An Evolutionary Perspective on Male Aggression against Females. Cambridge, MA: Harvard University Press, 2009.
Nunn, C. L. The number of males in primate social groups: A comparative test of the socioecological model. Behavioral Ecology and Sociobiology 46, 1-13 (1999).
Ostner, J. et al. Female reproductive synchrony predicts skewed paternity across primates. Behavioral Ecology 19, 1150-1158 (2008).
Palombit, R. A. et al. The adaptive value of 'friendships' to female baboons: Experimental and observational evidence. Animal Behaviour 54, 599-614 (1997).
Plavcan, J-M. "Sexual selection, measures of sexual selection, and sexual dimorphism in primates," in Sexual Selection in Primates, eds. P. M. Kappeler & C. P. van Schaik (Cambridge, UK: Cambridge University Press, 2004) 230-252.
Port, M. & Kappeler, P. M. The utility of reproductive skew models in the study of male primates, a critical evaluation. Evolutionary Anthropology 19, 46-46 (2010).
Port, M. et al. Costs and benefits of multi-male associations in redfronted lemurs (Eulemur fulvus rufus). Biology Letters 6, 620-622 (2010).
Schülke, O. et al. Social bonds enhance reproductive success in male macaques. Current Biology 20, 2207-2210 (2010).
Setchell, J. M. et al. Stress, social behaviour, and secondary sexual traits in a male primate. Hormones and Behavior 58, 720-728 (2010).
Smuts, B. B. & Smuts, R. W. Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Advances in the Study of Behavior 22, 1-63 (1993).
Soulsbury, C. D. Genetic patterns of paternity and testes size in mammals. PLoS ONE 5, e9581 (2010).
Trivers, R. L. "Parental investment and sexual selection," in Sexual Selection and the Descent of Man 1871-1971, ed. B. Campbell (London, UK: Heinemann, 1972) 136-179.
van Belle, S. et al. Social and hormonal mechanisms underlying male reproductive strategies in black howler monkeys (Alouatta pigra). Hormones and Behavior 56, 355-363 (2009).
van Schaik, C. P. "Infanticide by male primates: The sexual selection hypothesis revisited," In Infanticide by Males and Its Implications, eds. C. P. van Schaik & C. H. Janson (Cambridge, UK: Cambridge University Press, 2000) 27-60.
Vigilant, L. et al. Paternity and relatedness in wild chimpanzee communities. Proceedings of the National Academy of Sciences of the United States of America 98, 12890-12895 (2001).
Wroblewski, E. E. et al. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behaviour 77, 873-885 (2009).