« Prev Next »
T.H. Huxley wrote that to look upon other apes is to see “blurred copies of [one]self” (1863 pg. 73). The close physical relationship among apes, or hominoids, had already been recognized a century earlier by Carolus Linnaeus (1759). Living hominoids are united by features related to habitual orthogrady and below-branch behaviors: broad torsos with widely-spaced shoulder joints, stiff backs, long forelimbs, mobile limb joints, strong grasping ability, and the absence of a tail (Huxley, 1863; Harrison, 1987 & 1991; Shoshani et al., 1996). Humans lack several of these features, such as relatively long arms, as a result of secondary adaptation to bipedalism. Cranial attributes also unite hominoids, including wide anterior palates and relatively large brains (Jerison, 1973; Rae, 1997). Hominoids also have extended life spans with delayed maturation (Kelley, 1997), and occupy similar ecological niches - all species but humans inhabit tropical forests and rely significantly on ripe fruit and leaves for food (Fleagle, 2013).
Hylobatids (gibbons and siamangs) are smaller than other living apes and are distinct in other ways, reflecting their long period of evolutionary isolation from hominids (great apes and humans), which share a more recent common ancestor (Figure 1). Hominids (except humans) have anterior palates that are widened beyond those of hylobatids (Rae, 1997), possess even shorter, stiffer lower backs, and lack anatomical specializations for ricochetal brachiation (Young, 2003).
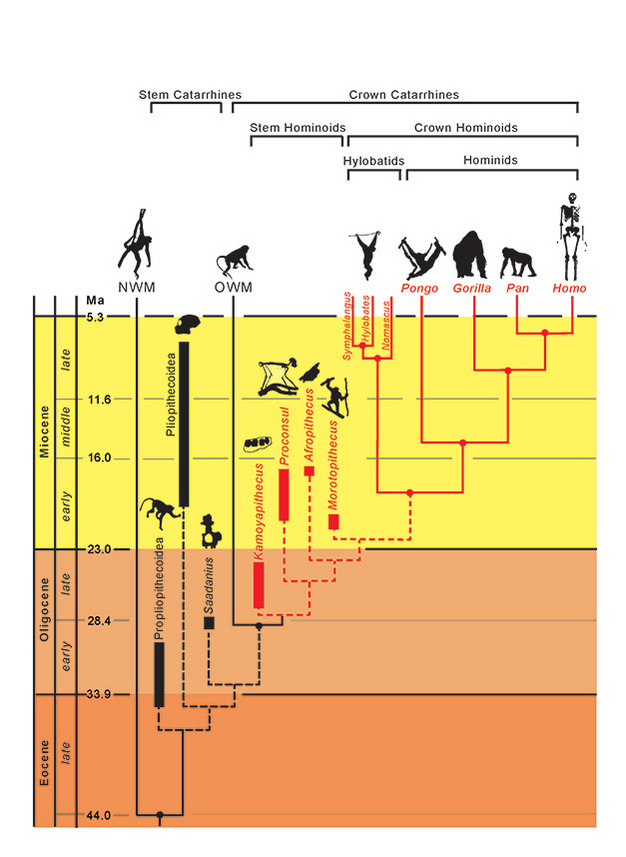
The phylogenetic relationships for taxa are represented by solid (extant) and dashed (extinct) lines. Geologic time is represented on the vertical axis. The timing of branching events for taxa is approximate only and inferred from a combination of molecular and fossil data (Steiper et al., 2004; MacLatchy et al., 2006; Steiper and Young, 2008 and 2009; Chan et al., 2010; Stevens et al., 2013). The phylogenetic placements of the fossil taxa are hypothetical, as discussed in the text. The position of Afropithecus and Morotopithecus could be reversed, or the two could be sister taxa. NMW refers to New World monkeys and OWM refers to Old World monkeys, while Propliopithecoidea, Pliopithecoidea and Saadanius refer to stem catarrhines from Afro-Arabia lacking any crown catarrhine synapomorphies.
Given these adaptive similarities among living hominoids, it is of some interest to investigate which (if any) characterized the initial divergence of hominoids from other catarrhine primates, notably the cercopithecoids (Old World monkeys). Combined morphological and genetic evidence place the hominoid-cercopithecoid divergence at 29.6 Ma (~24-38 Ma), and the hylobatid-hominid divergence at 18.8 Ma (~16-24 Ma) (Steiper and Young, 2009). Hominoids should therefore be present by the Oligocene, but how can we recognize them? Have we found them? Identifying these early fossil apes is potentially hindered by the following problems:
1) A deficient fossil record. There are few Oligocene sites in Africa that represent the appropriate time period, and although there are several early Miocene sites, many of their catarrhine taxa are poorly represented, particularly in the postcranium.
2) The length of time since the origin of Hominoidea. Basal members of a taxon may bear little resemblance to extant forms because they often retain numerous primitive features. For example, a very early ape could have been pronograde even if the last common ancestor of living apes had evolved adaptations for upright posture. Potential synapomorphies between stem and crown hominoids are thus expected to be few.
3) The long periods of evolution in extant lineages may have been individually unique such that modern taxa retain few defining synapomorphies, or some of their presumed synapomorphies may be parallelisms.
4) The evolutionary history of morphological transformation may not be appropriately resolved, leading to conflicting phylogenetic interpretations.
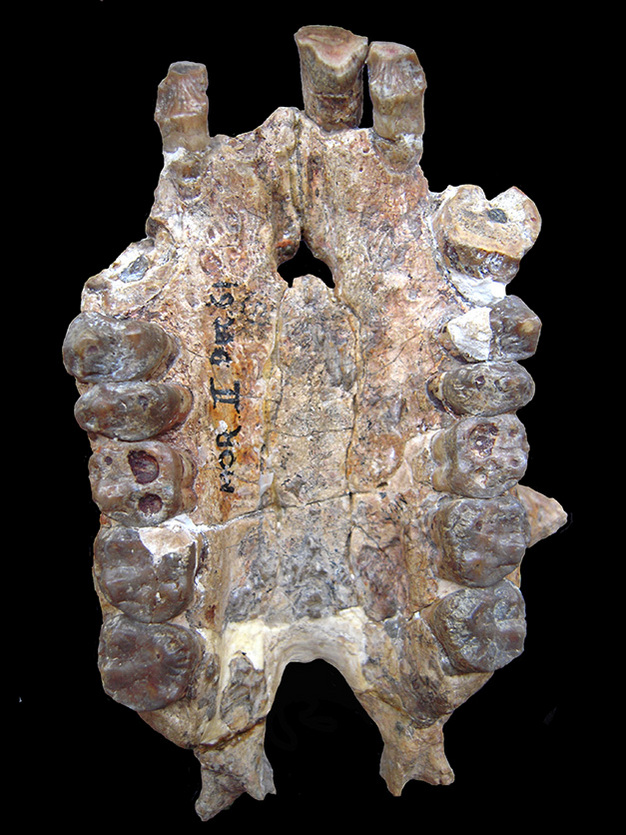
Comparative study both involving stem and crown catarrhines can mitigate these issues (Zalmout et al., 2010). For example, features present in a late Oligocene or early Miocene species, which are not shared primitively with stem catarrhines and cercopithecoids, but are observed in crown hominoids, are plausible hominoid synapomorphies. For example, the ethmo-frontal sinus occurs in African apes and is absent in Afro-Arabian stem catarrhines, cercopithecoids, Pongo, and Hylobates (Rossie et al., 2002; Rossie, 2008). The sinus also occurs in three early Miocene putative hominoids: Proconsul, Morotopithecus and Afropithecus (ibid.). The distribution of this trait can thus be interpreted as a derived character first occurring in stem hominoids, and lost in Asian apes (Begun, 1992; Moyà-Solà and Köhler, 1995; but see Rossie, 2008, for alternate ways to interpret this character). Another cranial feature, increased cross-sectional robustness of the canines, and concomitant expansion of the anterior palate, has a similar distribution (i.e., it is found in extant hominids (lost secondarily in humans) as well as in Proconsul, Morotopithecus and Afropithecus, but is absent in stem catarrhines and cercopithecoids), and also supports the interpretation that the Miocene taxa are hominoids (Begun et al., 1997; Zalmout et al., 2010). The late Oligocene Kamoyapithecus is known only from gnathodental remains (Leakey et al., 1995), but the presence of cross-sectionally expanded upper canine teeth could signal that it is also a hominoid (Zalmout et al., 2010). In addition, these four fossil genera include species with estimated body sizes in excess of 30 kg, the first catarrhines to attain this size.
Given the large size and arboreal niche of the extant apes (gibbons are probably secondarily dwarfed: Tyler, 1993), large body size may also be a stem hominoid characteristic. In crown hominoids, this trait is traditionally tied to hominoid exploitation of high quality arboreal foods, enabled by locomotor innovations including enhanced limb mobility and grasping ability (which allow weight to be distributed across multiple supports), and orthogrady (Avis, 1962; Napier, 1967; Tuttle, 1975; Fleagle, 1976; Cachel, 1979; Temerin and Cant, 1983; Cant, 1987; Wheatley, 1987; Kelley, 1997; MacLatchy, 2004).
Evidence for these postcranial transformations is variably expressed in Proconsul, Afropithecus and Morotopithecus. Proconsul is well known from several partial skeletons and has been reconstructed as an above-branch quadruped, with a pronograde trunk, but seems to have lacked a tail (Napier and Davis, 1959; Beard et al., 1986; Ward et al., 1991; Ward, 1998). Proconsul is also is reconstructed to have enhanced grasping ability, and greater mobility in joints such as the elbow, relative to primitive catarrhines and cercopithecoids, as evidenced by features such as a distinct zona conoidea in the distal humerus (Rose, 1988). Afropithecus is less well represented postcranially, but resembles Proconsul in known elements (Ward, 1998). Although the association between the cranial and postcranial samples from the Moroto localities is the subject of disagreement (Senut et al., 2000), the postcranial remains of Morotopithecus differ consistently from those attributed to Proconsul, suggesting instead a hominoid with a stiff lower back, mobile gleno-humeral joint, and femur adapted for deliberate climbing, more reminiscent of crown hominoids (MacLatchy et al., 2000).
In addition to larger body sizes, all taxa that can be evaluated have pronounced sexual dimorphism in both body size and canine morphology (Kelley, 1986; Ruff et al., 1989). While strong sexual dimorphism is a primitive catarrhine feature, it is further elaborated in apparent stem hominoids and modern great apes (but secondarily reduced in humans).
There are few, if any, compelling synapomorphies in the hominoid dentition. Because hominoids retain primitive catarrhine tooth morphology (Kay and Ungar, 1997), while cercopithecoid teeth share the clear-cut synapomorphy of molar bilophodonty, there is a history of using weakly diagnostic dental features to acknowledge that some poorly represented Oligocene and Miocene catarrhines might be hominoids, pending the discovery of more complete specimens (e.g. Andrews, 1978 and 1992; Stevens et al., 2013). Such Oligocene and Miocene catarrhines have been referred to informally as "dental apes" (e.g. Fleagle and Kay, 1987; Cartmill and Smith, 2009), or placed in taxonomic limbo using a designation such as Superfamily incertae sedis (e.g. Begun, 2007).
Notably, the idea has been advanced that none of the fossil taxa discussed above is a hominoid, and that the first recognizable hominoid is the 14-million-year-old Kenyapithecus, which has some derived craniodental attributes (e.g., reduced molar cingulum, a higher position of origin of the zygomatic arch) relative to early Miocene forms (Harrison, 2010). If so, there would be an inexplicable gap of at least 10 Ma - despite a robust fossil record through much of this time period - between the estimated origin of hominoids and their first appearance.
Although paleoanthropologists are unable to determine the phylogenetic placement of “dental apes” or Kamoyapithecus with confidence, there is broad support for the hypothesis that Proconsul, Afropithecus and Morotopithecus are stem hominoids, rather than stem catarrhines. As a window into early hominoid evolution, these taxa suggest the following:
1) Large body size, enlargement of the anterior dentition, and subtle changes in the posterior dentition (e.g., more elongated molars and changes in molar cusp placement and cingulum development) suggest occupation of fundamentally different ecological niches than those of stem catarrhines. Large canines function in both food procurement and in sexual selection, with pronounced dimorphism suggesting the latter was a factor. Large size allows access to new foods and improves foraging flexibility (Wheatley, 1987), decreases predation risks, promotes survivorship and, coupled with factors such as habitat stability, may be linked to delayed maturation. Analysis of the dental development pattern of Afropithecus (and possibly Proconsul) suggests delayed maturation relative to other anthropoids (Kelley, 1997, 2002), making slow life history another possible stem hominoid synapomorphy.
2) By the early Miocene, there is at least modest locomotor and dietary diversity among the large-bodied probable stem hominoids, indicating the beginnings of a true adaptive radiation, with increasing body size, increasing locomotor novelty and more specialized exploitation of arboreal food sources evolving concurrently.
3) It is not currently resolvable which anatomical features associated with orthogrady and joint mobility in modern apes are homologous or homoplastic (e.g., Harrison, 1991; Larson, 1998; Young, 2003; MacLatchy, 2004 & 2010; Lovejoy et al., 2009). This debate has implications for interpreting the evolutionary position of purported stem hominoids, and for reconstructing the pattern and timing of the emergence of modern ape adaptations.
For example, postcranial similarities between Morotopithecus and living apes could be interpreted a number of ways. If the living apes acquired their orthogrady through parallel evolution, then Morotopithecus represents the earliest known instance of a transition that occurred several times in hominoid evolution. This is of great adaptive interest, suggesting that some underlying developmental or genetic propensity (see below) kept steering the hominoid evolutionary response in the same direction. It would also mean certain postcranial traits must be used cautiously in phylogenetic analysis (Sanders and Bodenbender, 1994). On the other hand, if these locomotor similarities were inherited from a common ancestor, Morotopithecus would be more closely related to crown hominoids than Proconsul (and possibly Afropithecus). Yet another possibility is that Morotopithecus was an independent "experiment," and that the common ancestor of crown hominoids was nevertheless orthograde.
4) As discussed above, despite differences in locomotor specializations and long periods of independent evolution, all surviving hominoid taxa share a similar body plan that differentiates them from other primates (with the exception of some ateline monkeys, which have convergently evolved torsos and forelimb specializations similar to those of apes (Erikson, 1963; Larson, 1998). The basic anatomical underpinnings of the ape body plan have led to remarkable locomotor diversity and versatility, enabling large bodied primates to move effectively arboreally (through climbing and suspension) and terrestrially (using knuckle-walking and bipedalism). Such locomotor innovation, tied to features such as orthogrady, likely underlies the evolutionary "success" of all living hominoids, and seems to have been present to some degree in stem hominoids. Developmental work by Young and colleagues (2010) has shown that extant apes have reduced integration between the fore- and hindlimbs compared to quadrupedal monkeys. Relaxation of this genetic constraint has been interpreted to have allowed the limbs to evolve more freely in different ways in response to selection pressures (ibid.). Inferred differential limb use in Morotopithecus (MacLatchy et al., 2013) and younger Miocene taxa (e.g. Moya Sola and Kohler, 1996; Moya Sola et al., 2004; Nakatsukasa and Kunimatsu, 2009) provisionally supports Young and colleagues' (2010) supposition that limb "evolvability" may be an ancestral hominoid trait, but more fossil material and a better understanding of the genetics underlying this trait, are needed.
Future insight into hominoid origins and early evolution will emerge from continued attempts to identify and understand the basis of possible hominoid synapomorphies. This, in turn, will require an improved fossil record for both stem and crown catarrhines, and more detailed anatomical, developmental and genetic study of the shared characters under scrutiny. Integrated study of fossil hominoids in their paleoenvironmental context may also help us better understand why hominoid features were selected for, whether once or multiple times.
Glossary
ateline- any member of subfamily Atelinae, the group of suspensory, South American monkeys comprised of woolly monkeys, woolly spider monkeys, spider monkeys, and their closest extinct relatives
bilophodonty - a molar feature in Old World monkeys in which the front (mesial) and back (distal) pairs of cusps are connected by ridges or lophs
below-branch behaviors - locomotor or postural behaviors in which the body is suspended under a substrate by the hands, feet or tail
body plan - the anatomical blueprint of an organism or group of organisms
catarrhines - any member of Infraorder Catarrhini, the group of anthropoids comprised of apes, Old World monkeys and their extinct relatives
cercopithecoid - any member of Superfamily Cercopithecoidea which is comprised of the Old World monkeys
character polarity - determining the evolutionary sequence in which a trait transformed from a primitive (plesiomorphic or ancestral) character state to a derived (apomorphic) character state
cingulum - a ridge or shelf of enamel that encircles some portion of the base (as in canines or incisors) or crown margin (as in molars) of a tooth
crown group - a group with more derived features that has evolved from a stem group; often, but not exclusively, extant.
ethmo-frontal sinus - an epithelially lined cavity of the ethmoid bone that extends into the frontal bone
hominid - any member of Family Hominidae, comprised of orang-utans, gorillas, chimpanzees, bonobos and humans, as well as their extinct relatives
Hominoidea - one of two crown catarrhine superfamilies, comprised of hylobatids, hominids and any extinct forms that are more closely related to hylobatids and hominids than other catarrhines
homologous - defines a character state that is inherited from an ancestor
homoplastic - defines a character state that has independently evolved two or more times, and therefore does not have a unique origin
Hylobates - the genus name of a gibbon
hylobatid - any member of Family Hylobatidae, comprised of gibbons, siamangs and their extinct relatives
Miocene - The geological epoch between 23 and 5.3 million years ago
Oligocene - the geological epoch between 33.9 and 23 million years ago
Orthogrady - upright trunk posture associated with suspension, vertical climbing, bipedalism, etc.
Pan - the genus name of the chimpanzee and the bonobo
Parallelism - when a feature has evolved independently in related lineages, often inferred to be through the alteration of similar developmental pathways
Phylogenetic - having to do with the evolutionary history, especially the branching relationships, of organisms
Pitheciinae - a group of medium-sized New World Monkeys that specialize in eating fruits and seeds with tough outer coverings ("sclerocarps")
Pongo - the genus name of the orangutan
postcranium - all of the body inferior to the skull
pronograde - a posture in which the trunk is positioned approximately horizontally
quadrupedal - a form of habitual locomotion in which all four limbs are weight-bearing, of generally similar length, and positioned below a more-or-less horizontally-oriented trunk
richochetal brachiation - rapid, hand-over-hand suspensory locomotion that includes a phase in which neither hand is contacting a substrate
sexual dimorphism - differences in the size or shape of body parts between males and females due to sexual selection
sexual selection - differential reproduction resulting from differences in the ability to obtain mates
stem group - an ancestral group with more primitive characters than the crown group; often, but not exclusively, extinct.
synapomorphy - a derived character state shared by multiple lineages. To be contrasted with symplesiomorphy: a primitive condition shared by multiple lineages.
zona conoidea - a gutter adjacent to the capitulum on the distal humerus that serves to buttress the margin of the proximal radius during forearm rotation.
zygomatic arch - The "cheekbone" or arched bony process of the skull that receives contributions from the maxilla, zygomatic and temporal bones
References and Recommended Reading
Andrews, P. A revision of the Miocene Hominoidea of East Africa. Bulletin of the British Museum (Natural History) 30, 85-224 (1978).
Andrews, P. Evolution and Environment in the Hominoidea. Nature 360, 641-646 (1992).
Avis, V. Brachiation: The crucial issue for man's ancestry. Southwestern Journal of Anthropology 18, 119-148 (1962).
Beard, K. C., Teaford, M. F. & Walker, A. New wrist bones of Proconsul africanus and P. nyanzae from Rusinga Island, Kenya. Folia Primatologica 47, 97-118 (1986).
Begun, D. R. Miocene fossil hominids and the chimp-human clade. Science 257, 1929-1933 (1992).
Begun D. R., Teaford M. F., Walker A. Comparative and functional anatomy of Proconsul phalanges from the Kaswanga primate site, Rusinga Island, Kenya. Journal of Human Evolution 26, 89-165 (1994).
Begun, D. R. & Kordos, L. Phyletic affinities and functional convergence in Dryopithecus and other Miocene and living hominids. In Function, Phylogeny, and Fossils: Miocene Hominoid Evolution and Adaptations. eds. Begun, D. R., Ward, C. V. & Rose, M. D. (New York: Plenum Press, 1997) 291-316.
Boschetto, H. B., Brown, F. H. & McDougall, I. Stratigraphy of the Lothidok range, northern Kenya, and K/AR ages of its Miocene primates. Journal of Human Evolution 22, 47-71 (1992).
Cachel, S. Paleoecological model for the origin of higher primates. Journal of Human Evolution 8, 351-359 (1979).
Cant, J. G. H. Positional behavior of female Bornean orangutans (Pongo pygmaeus). American Journal of Primatology 12, 71-90 (1987).
Cartmill, M., Smith, F. The Human Lineage. Hobeken: Wiley Blackwell (2009)
Chan, Y.-C., Roos, C., Inoue-Murayama, M., Inoue, E., Shih, C.-C., Jai-Chyi, K. Pei, K.J.-C., Vigilant, L. Mitochondrial Genome Sequences Effectively Reveal the Phylogeny of Hylobates Gibbons. PLoSONE 5, e11419 (2010).
Collard, M. & Wood, B. How reliable are current estimates of fossil catarrhine phylogeny? An assessment using extant great apes and Old World monkeys. In Phylogeny of Eurasian Neogene Hominoid Primates Vol. 2. eds. de Bonis, L. & Koufos, G. D. (Cambridge: Cambridge University Press, 2001) 118-150.
Drake, R. E., Van Couvering, J. A., et al. New chronology for the early Miocene mammalian faunas of Kisingiri, western Kenya. Journal of the Geological Society, London 145, 479-491 (1988).
Erikson, G. E. Brachiation in New World monkeys and in anthropoid apes. Symposia of the Zoological Society of London 10, 135-164 (1963).
Fleagle, J. G. Locomotion and posture of Malayan siamang and implications for hominoid evolution. Folia Primatologica 26, 245-269 (1976).
Fleagle, J.G. Primate Adaptation and Evolution, Third Edition. San Diego: Academic Press (2013).
Fleagle, J.G., Kay, R.F. New interpretations of the phyletic position of Oligocene hominoids. In New Interpretations of Ape and Human Ancestry. eds. Ciochon, R.L. & Corruccini, R.S. (New York: Plenum Publishing 1987) 181-210.
Gebo, D. L., MacLatchy, L. et al. A hominoid genus from the early Miocene of Uganda. Science 276, 401-404 (1997).
Gibbs, S., Collard, M. & Wood, B. Soft-tissue characters in higher primate phylogenetics. Proceedings of the National Academy of Sciences, USA 97, 11130-11132 (2000).
Harrison, T. The phylogenetic relationships of the early catarrhine primates: A review of the current evidence. Journal of Human Evolution 16, 41-80 (1987).
Harrison, T. A taxonomic revision of the small catarrhine primates from the Early Miocene of East Africa. Folia Primatologica 50, 59-108 (1988).
Harrison, T. The implications of Oreopithecus bambolii for the origins of bipedalism. In Origine(s) de la Bipedie chez les Hominides. eds. Coppens, Y. & Senut, B. (Paris: Cahiers de Paleoanthropologie 1991) 235-244.
Harrison, T. Evidence for a tail in Proconsul heseloni. American Journal of Physical Anthropology, 93-94 (1998).
Harrison, T. Late Oligocene to middle Miocene catarrhines from Afro-Arabia. In The Primate Fossil Record. ed. Hartwig. W. C. (New York: Cambridge University Press, 2002). 311-338
Harrison, T. & Andrews, P. The anatomy and systematic position of the early Miocene proconsulid from Meswa Bridge, Kenya. Journal of Human Evolution 56, 479-496 (2009).
Harrison, T. Dendropithecoidea, Proconsuloidea, and Hominoidea. In Cenozoic Mammals of Africa. eds. Werdelin, L. & Sanders, W. J. (Berkeley: University of California Press, 2010) 429-469.
Huxley, T. H. Evidence as to Man's Place in Nature. New York: D. Appleton and Company (1863).
Jerison, H. J. Brain evolution and biological intelligence. Bulletin of the Psychonomic Society 2, 339 (1973).
Kelley, J., Species recognition and sexual dimorphism in Proconsul and Rangwapithecus. Journal of Human Evolution 15, 461-495 (1986).
Kelley, J. Paleobiological and phylogenetic significance of life history in Miocene Hominoids. In Function, Phylogeny, and Fossils: Miocene Hominoid Evolution and Adaptations. eds. Begun, D. R., Ward, C. V. & Rose, M. D. (New York: Plenum Press, 1997) 173-208.
Kelley, J. The hominoid radiation in Asia. In The Primate Fossil Record. ed. Hartwig, W. C. (New York: Cambridge University Press, 2002) 369-384.
Larson, S. G. Parallel evolution in the hominoid trunk and forelimb. Evolutionary Anthropology 6, 87-99 (1998).
Leakey R. E., Leakey M. G., Walker A. C. Morphology of Afropithecus turkanensis from Kenya. American Journal of Physical Anthropology 76, 289-307 (1988).
Leakey, M. G., Ungar, P. S. & Walker, A. A new genus of large primate from the Late Oligocene of Lothidok, Turkana District, Kenya. Journal of Human Evolution 28, 519-531 (1995).
Leakey, M. & Walker, A. Afropithecus: Function and phylogeny. In Function, Phylogeny, and Fossils: Miocene Hominoid Evolution and Adaptations. eds. Begun, D. R., Ward, C. V., & Rose, M. D. 225-239 (New York: Plenum Press, 1997).
Linnaeus, C. Systema Naturae, Tenth Edition. New York: Weldon & Wesley (1759).
Lovejoy, C. O., Suwa, G., Simpson, S. W., Matternes, J. H. & White, T. D. The Great Divides: Ardipithecus ramidus Reveals the Postcrania of Our Last Common Ancestors with African Apes. Science 326, 100-106 (2009).
MacLatchy, L., Gebo, D., et al. Postcranial functional morphology of Morotopithecus bishopi, with implications for the evolution of modern ape locomotion. Journal of Human Evolution 39, 159-183 (2000).
Maclatchy, L. The oldest ape. Evolutionary Anthropology 13, 90-103 (2004).
Maclatchy, L., Deino, A. & Kingston, J. An updated chronology for the early Miocene of NE Uganda. Journal of Vertebrate Paleontology 26, 93A-93A (2006).
MacLatchy, L. & DeSilva, J. The postcranial anatomy of Proconsul major. Journal of Vertebrate Paleontology 29, 139A (2009).
MacLatchy, L. Morotopithecus. In 2010 McGraw-Hill Yearbook of Science and Technology. 245-248 (New York: McGraw-Hill, 2010).
MacLatchy, L., Rossie, J., Smith, T. & Tafforeau, P. Evidence for dietary niche separation in the Miocene hominoids Morotopithecus and Afropithecus. American Journal of Physical Anthropology Supplement 48, 181 (2010).
MacLatchy, L., Kingston, J. and Kityo, R. The femur of Morotopithecus. Journal of Vertebrate Paleontology in press.
Moyà-Solà S, Köhler M. New partial cranium of Dryopithecus lartet, 1863 (Hominoidea, Primates) from the upper Miocene of Can Llobateres, Barcelona, Spain. Journal of Human Evolution 29, 101-139 (1995).
Napier, J. R. & Davis, P. R. The forelimb skeleton and associated remains of Proconsul africanus. Fossil Mammals of Africa 16, 1-69 (1959).
Napier, J. R. Evolutionary aspects of primate locomotion. American Journal of Physical Anthropology 27, 333-342 (1967).
Peppe, D. J., McNulty, K. P. et al. Stratigraphic interpretation of the Kulu Formation (Early Miocene, Rusinga Island, Kenya) and its implications for primate evolution. Journal of Human Evolution 56, 447-461 (2009).
Peppe, D. J., Deino, A. L. et al. New age constraints on the early Miocene faunas from Rusinga and Mfangano Islands (Lake Victoria, Kenya). American Journal of Physical Anthropology 144, 237-237 (2011).
Pickford, M. & Andrews, P. The Tinderet Miocene sequence in Kenya. Journal of Human Evolution 10, 11-33 (1981).
Rae, T. C. The early evolution of the hominoid face. In Function, Phylogeny, and Fossils: Miocene Hominoid Evolution and Adaptations. eds. Begun, D. R., Ward, C. V. & Rose, M. D. (New York: Plenum Press, 1997) 59-77.
Rae, T. C. Mosaic evolution in the origin of the hominoidea. Folia Primatologica 70, 125-135 (1999).
Rasmussen, D. T. & Gutiérrez, M. A Mammalian Fauna from the late Oligocene of northwestern Kenya. Palaeontographica Abteilung a-Palaozoologie-Stratigraphie 288, 1-52 (2009).
Rose, M. D. Miocene hominoid postcranial morphology: Monkey-like, ape-like, neither or both? In New Interpretations of Ape and Human Ancestra. eds. Ciochon, R. L. & Corruccini, R. S. (New York: Plenum Press, 1983) 405-417.
Rose, M. D. Another look at the anthropoid elbow. Journal of Human Evolution 17, 193-224 (1988).
Rose, M. D. Kinematics of the trapezium-1st metacarpal joint in extant anthropoids and Miocene hominoids. Journal of Human Evolution 22, 255-266 (1992).
Rossie, J. B., Simons, E. L. et al. Paranasal sinus anatomy of Aegyptopithecus: implications for hominoid origins. Proceedings of the National Academy of Sciences USA. 99, 8454-8456 (2002).
Rossie, J. B. The Phylogenetic Significance of Anthropoid Paranasal Sinuses. Anatomical Record 291, 1485-1498, (2008).
Ruff, C.B., Walker, A., Teaford, M.F. Body mass, sexual dimorphism and femoral proportions ofProconsul from Rusinga and Mfangano Islands, Kenya 18, 515-536 (1989).
Sanders, W. J. & Bodenbender, B. E. Morphometric analysis of lumbar vertebra UMP 67-28: implications for spinal function and phylogeny of the Miocene Moroto hominoid. Journal of Human Evolution 26, 203-237 (1993).
Senut, B., Pickford, M., Gommery, D., Kunimatsu, Y. Un nouveau genre d'hominoïde du Miocène inférieur d'Afrique orientale : Ugandapithecus major (Le Gros Clark & Leakey, 1950). C. R. Acad. Sci. Paris, Sciences de la Terre et des planètes 331, 227-233 (2000).
Shoshani, J., Groves, C. P., et al. Primate phylogeny: morphological vs molecular results. Molecular Phylogenetics and Evolution 5, 102-154 (1996).
Steiper, M. E., Young, N. M. & Sukarna, T. Y. Genomic data support the hominoid slowdown and an Early Oligocene estimate for the hominoid-cercopithecoid divergence. Proceedings of the National Academy of Sciences USA 101, 17021-17026 (2004).
Steiper, M. E. & Young, N. M. Timing primate evolution: Lessons from the discordance between molecular and paleontological estimates. Evolutionary Anthropology 17, 179-188 (2008).
Steiper, M. E. & Young, N. M. Primates. In The Timetree of Life. eds. Hedges, S. B. & Kumar, S. (Oxford: Oxford University Press, 2009). 482-486.
Temerin, L. A. & Cant, J. G. H. The evolutionary divergence of Old World monkeys and apes. American Naturalist 122, 335-351 (1983).
Tuttle, R. H. Parallelism, brachiation, and hominoid phylogeny. In Phylogeny of the Primates: A Multidisciplinary Approach. eds. T. J. Luckett & F. Szalay (New York: Plenum Press, 1975) 447-480.
Tyler, D. E. The evolutionary history of the gibbon. In Evolving Landscapes and Evolving Biotas of East Asia since the mid-Tertiary ed. Jablonski, N. (Hong Kong: Center for Asian Studies, Hong Kong University, 1993). 228-240.
Walker, A. Proconsul function and phylogeny; In Function, Phylogeny, and Fossils: Miocene Hominoid Evolution and Adaptations. eds. Begun, D. R., Ward, C. V. & Rose, M. D. (New York: Plenum Press, 1997) 209-224.
Ward, C. V., Walker, A. & Teaford, M. F. Proconsul did not have a tail. Journal of Human Evolution 21, 215-220 (1991).
Ward, C. V., Walker, A. & Teaford, M. F. Still no evidence for a tail in Proconsul heseloni. American Journal of Physical Anthropology, 273-273 (1999).
Ward, C. V. Afropithecus, Proconsul, and the primitive hominoid skeleton. In Primate Locomotion. eds. Strasser, E., Fleagle, J., McHenry, H. & Rosenberger, A. (New York: Plenum Press, 1998) 337-352.
Ward, C. V. Postcranial and locomotor adaptations of hominoids in Handbook of Paleoanthropology Vol. 2. eds. Henke, W. & Tattersall, I. (Heidelberg: Springer, 2007). 1011-1030.
Wheatley, B. P. The evolution of large body size in orangutans: a model for hominoid divergence. American Journal of Primatology 13, 313-324 (1987).
Young, N. A reassessment of living hominoid postcranial variability: implications for ape evolution. Journal of Human Evolution 45, 441-464 (2003).
Young, N. M., Wagner, G. P. & Hallgrimsson, B. Development and the evolvability of human limbs. Proceedings of the National Academy of Sciences USA 107, 3400-3405 (2010).
Zalmout, I. S. et al. New Oligocene primate from Saudi Arabia and the divergence of apes and Old World monkeys. Nature 466, 360-364 (2010).