« Prev Next »
Introduction
Eutrophication is characterized by excessive plant and algal growth due to the increased availability of one or more limiting growth factors needed for photosynthesis (Schindler 2006), such as sunlight, carbon dioxide, and nutrient fertilizers. Eutrophication occurs naturally over centuries as lakes age and are filled in with sediments (Carpenter 1981). However, human activities have accelerated the rate and extent of eutrophication through both point-source discharges and non-point loadings of limiting nutrients, such as nitrogen and phosphorus, into aquatic ecosystems (i.e., cultural eutrophication), with dramatic consequences for drinking water sources, fisheries, and recreational water bodies (Carpenter et al. 1998). For example, aquaculture scientists and pond managers often intentionally eutrophy water bodies by adding fertilizers to enhance primary productivity and increase the density and biomass of recreationally and economically important fishes (Figure 1) via bottom-up effects on higher trophic levels (Boyd & Tucker 1998). However, during the 1960s and 1970s, scientists linked algal blooms to nutrient enrichment resulting from anthropogenic activities such as agriculture, industry, and sewage disposal (Schindler 1974). The known consequences of cultural eutrophication include blooms of blue-green algae (i.e., cyanobacteria, Figure 2), tainted drinking water supplies, degradation of recreational opportunities, and hypoxia. The estimated cost of damage mediated by eutrophication in the U.S. alone is approximately $2.2 billion annually (Dodds et al. 2009).


Consequences
The most conspicuous effect of cultural eutrophication is the creation of dense blooms of noxious, foul-smelling phytoplankton that reduce water clarity and harm water quality (Figure 2). Algal blooms limit light penetration, reducing growth and causing die-offs of plants in littoral zones while also lowering the success of predators that need light to pursue and catch prey (Lehtiniemi et al. 2005). Furthermore, high rates of photosynthesis associated with eutrophication can deplete dissolved inorganic carbon and raise pH to extreme levels during the day. Elevated pH can in turn ‘blind' organisms that rely on perception of dissolved chemical cues for their survival by impairing their chemosensory abilities (Figure 3) (Turner & Chislock 2010). When these dense algal blooms eventually die, microbial decomposition severely depletes dissolved oxygen, creating a hypoxic or anoxic ‘dead zone' lacking sufficient oxygen to support most organisms. Dead zones are found in many freshwater lakes including the Laurentian Great Lakes (e.g., central basin of Lake Erie; Arend et al. 2011) during the summer. Furthermore, such hypoxic events are particularly common in marine coastal environments surrounding large, nutrient-rich rivers (e.g., Mississippi River and the Gulf of Mexico; Susquehanna River and the Chesapeake Bay) and have been shown to affect more than 245,000 square kilometers in over 400 near-shore systems (Diaz & Rosenberg 2008). Hypoxia and anoxia as a result of eutrophication continue to threaten lucrative commercial and recreational fisheries worldwide.

Some algal blooms pose an additional threat because they produce noxious toxins (e.g., microcystin and anatoxin-a; Chorus and Bartram 1999). Over the past century, harmful algal blooms (HABs) have been linked with (1) degradation of water quality (Francis 1878), (2) destruction of economically important fisheries (Burkholder et al. 1992), and (3) public health risks (Morris 1999). Within freshwater ecosystems, cyanobacteria are the most important phytoplankton associated with HABs (Paerl 1988). Toxigenic cyanobacteria, including Anabaena, Cylindrospermopsis, Microcystis, and Oscillatoria (Planktothrix), tend to dominate nutrient-rich, freshwater systems due to their superior competitive abilities under high nutrient concentrations, low nitrogen-to-phosphorus ratios, low light levels, reduced mixing, and high temperatures (Downing et al. 2001; Paerl & Huisman 2009; Paerl and Paul 2012). Poisonings of domestic animals, wildlife (Figure 4), and even humans by blooms of toxic cyanobacteria have been documented throughout the world and date back to Francis' (1878) first observation of dead livestock associated with a bloom of cyanobacteria. Furthermore, cyanobacteria are responsible for several off-flavor compounds (e.g., methylisoborneal and geosmin) found in municipal drinking water systems as well as in aquaculture-rased fishes, resulting in large financial losses for state and regional economies (Crews & Chappell 2007). In addition to posing significant public health risks, cyanobacteria have been shown to be poor quality food for most zooplankton grazers in laboratory studies (Wilson et al. 2006; Tillmanns et al. 2008), thus reducing the efficiency of energy transfer in aquatic food webs and potentially preventing zooplankton from controlling algal blooms.

Eutrophication is also associated with major changes in aquatic community structure. During cyanobacterial blooms, small-bodied zooplankton tend to dominate plankton communities, and past observational studies have attributed this pattern to anti-herbivore traits of cyanobacteria (e.g., toxicity, morphology, and poor food quality) (Porter 1977). However, the biomass of planktivorous fish is often positively related to nutrient levels and ecosystem productivity. Piscivorous fishes (e.g., bass, pike) tend to dominate the fish community of nutrient-poor, oligotrophic lakes, while planktivorous fishes (e.g., shad, bream) become increasingly dominant with nutrient enrichment (Jeppesen et al. 1997). Thus, an alternative explanation for the lack of zooplankton control of cyanobacterial blooms could include consumption of zooplankton by planktivores.
Controls
Water resource managers routinely employ a variety of strategies to minimize the effects of cultural eutrophication, including (1) diversion of excess nutrients (Edmondson 1970), (2) altering nutrient ratios (Downing et al. 2001), (3) physical mixing (Huisman et al. 2004), (4) shading water bodies with opaque liners or water-based stains, and (5) application of potent algaecides and herbicides (Boyd & Tucker 1998). In general, these strategies have proven to be ineffective, costly, and/or impractical, especially for large, complex ecosystems (but see Edmondson 1970). Water quality can often be improved by reducing nitrogen and/or phosphorus inputs into aquatic systems, and there are several well-known examples where bottom-up control of nutrients has greatly improved water clarity. However, nutrient reduction can be difficult (and expensive) to control, especially in agricultural areas where the algal nutrients come from nonpoint sources. Furthermore, in lakes where external loading of nutrients has been reduced, internal loading of nutrients from sediments may prevent improvements in water quality (Søndergaard et al. 2003). The use of algaecides, such as copper sulfate, is also effective at reducing HABs temporally (Boyd & Tucker 1998). However, algaecides are expensive to apply, do not control the primary cause of the problem (i.e., abundant resources for primary producers) and pose risks to humans, livestock, and wildlife, in addition to harming a variety of non-target aquatic organisms.
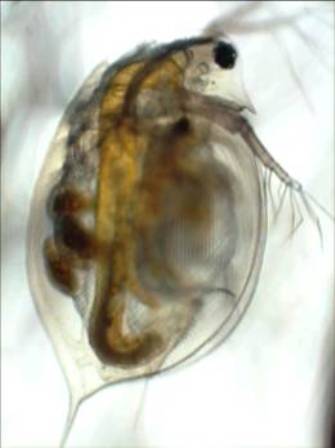
Another alternative for improving water quality in nutrient-rich lakes has been biomanipulation - the alteration of a food web to restore ecosystem health (Shapiro et al. 1975). The basic premise is that secondary consumers (planktivorous fishes) are removed either through the addition of tertiary consumers (piscivorous fishes) or harvesting, which allows for the dominance of large-bodied, generalist grazers (e.g., Daphnia) to control phytoplankton (Figure 5). When planktivorous fishes are abundant and there is no predation refuge (e.g., oxygenated hypolimnion) for large-bodied zooplankton, less efficient small-bodied zooplankton grazers (e.g., rotifers and herbivorous copepods) typically dominate zooplankton communities, thus allowing for the overgrowth of phytoplankton (i.e., algal bloom). Many past studies have shown strong correlations between the size structure of zooplankton communities and phytoplankton abundance. These data support the notion that predatory top-down forces can have important implications for aquatic communities and ecosystems. With that said, fish-centric biomanipulation effects on water quality are typically short-lived (i.e., weeks to months), most obvious in small, easily-managed systems (i.e., ponds), and impacted by resource availability, namely phosphorus and nitrogen (Benndorf 1990; Carpenter et al. 1995).
Conclusions
Glossary
anoxia: Lack of dissolved oxygen in water
biomanipulation: The alteration of a food web to restore ecosystem health Eutrophication - elevated primary production
HAB: Harmful algal bloom; abundant phytoplanktonhypoxia: Reduced dissolved oxygen concentration in water that stresses an organism
internal loading: Release of nutrients, such as phosphorus and nitrogen, from sediments during low oxygen concentration conditions
photosynthesis: Conversion of inorganic carbon (carbon dioxide) to organic carbon (glucose) by a primary producer
References and Recommended Reading
Arend, K. K. et al. Seasonal and interannual effects of hypoxia on fish habitat quality in central Lake Erie. Freshwater Biology 56, 366-383 (2011).
Benndorf, J. Conditions for effective biomanipulation - conclusions derived from whole-lake experiments in Europe. Hydrobiologia 200, 187-203 (1990).
Boyd, C. E. & Tucker, C. S. Pond aquaculture water quality management. Norwell MA: Kluwer (1998).
Burkholder J. M. et al. New 'phantom' dinoflagellate is the causative agent of major estuarine fish kills. Nature 358, 407-410 (1992).
Carpenter, S. R. Submersed vegetation: an internal factor in lake ecosystem succession. The American Naturalist 118, 372-383 (1981).
Carpenter, S. R. et al. Biological control of eutrophication in lakes. Environmental Science & Technology 29, 784-786 (1995).
Carpenter, S. R. et al. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8, 559-568 (1998).
Chorus, I. & Bartram, J. (Eds). Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. London UK: E & FN Spon (1999).
Crews, J. R. & Chappell, J. A. Agriculture and natural resources U.S. catfish industry outlook. Auburn AL: Auburn University (2007).
Diaz, R. J. & Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 321, 926-929 (2008).
Dodds, W. K. et al. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environmental Science and Technology 43, 12-19 (2009).
Downing, J. A. et al. Predicting cyanobacteria dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences 58, 1905-1908 (2001).
Edmondson, W. T. Phosphorus, nitrogen, and algae in Lake Washington after diversion of sewage. Science 169, 690-691 (1970).
Francis, G. Poisonous Australian lake. Nature 18, 11-12 (1878).
Huisman J. et al. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85, 2960-2970 (2004).
Jeppesen, E. et al. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342/343, 151-164 (1997).
Lehtiniemi, M. et al. Turbidity decreases anti-predator behaviour in pike larvae, Esox Lucius. Environmental Biology of Fishes 73, 1-8 (2005).
Morris, J. G. Harmful algal blooms: an emerging public health problem with possible links to human stress on the environment. Annual Review of Energy and the Environment 24, 367-390 (1999).
Paerl, H. W. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnology and Oceanography 33, 823-847 (1988).
Paerl, H. W. & Huisman, J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports 1, 27-37 (2009).
Paerl, H. W. & Paul, V. J. Climate change: links to global expansion of harmful cyanobacteria. Water Research 46, 1349-63 (2012).
Porter, K. G. The plant-animal interface in freshwater ecosystems. American Scientist 65, 159-170 (1977).
Schindler, D. W. Eutrophication and recovery in experimental lakes: implications for lake management. Science 174, 897-899 (1974).
Schindler, D. W. Recent advances in the understanding and management of eutrophication. Limnology and Oceanography 51, 356-363 (2006).
Shapiro, J. et al. Biomanipulation: An ecosystem approach to lake restoration. In Water quality management through biological control (pp. 85-96). Eds. Brezonik, P. L. & Fox, J. L. Gainesville, FL: University of Florida (1975).
Smith, V. H. & Schindler, D. W. Eutrophication science: where do we go from here? Trends in Ecology and Evolution 24, 201-207 (2009).
Søndergaard, M. et al. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506-509, 135-145 (2003).
Tillmanns, A. R. et al. Meta-analysis of cyanobacterial effects on zooplankton population growth rate: species-specific responses. Fundamental and Applied Limnology 171, 285-295 (2008).
Turner, A. M. & Chislock, M. F. Blinded by the stink: nutrient enrichment impairs the perception of predation risk by freshwater snails. Ecological Applications 20, 2089-2095 (2010).
Wilson, A. E. et al. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnology and Oceanography 51, 1915-1924 (2006).






























