« Prev Next »

Species exhibit tremendous variation in the extent of their geographic distributions, and such inconsistencies in rangesize occur even among closely related and/or ecologically similar species. The combined influences of abiotic factors, individual and population responses to habitat quality and availability, and species interactions restrain species from unchecked dispersal beyond their observed range margins.
Abiotic Factors
The most recognizable limitations to the geographic distribution of a species include insurmountable physical barriers, such as mountains, oceans, rivers, deserts, and, more recently, expansive human development. These obstacles directly prevent species from dispersal, especially in the cases of oceans for terrestrial species, land masses for marine species, and transitions between freshwater and marine systems for many aquatic organisms. Some species are also limited by physical barriers that emerge periodically, such as in areas characterized by frequent flooding, fires, or volcanic eruptions.
More commonly, however, the range limits of species are not imposed directly through physical barriers, but rather through spatial gradients in climatic variables. While species are often capable of physically traversing the distances separating ecosystems, gradients in such factors as temperature, soil and water chemistry, and precipitation present physiological barriers to dispersal. For example, the flat topographies of many deserts are not effective physical barriers to dispersal, but their exceedingly dry conditions prevent organisms with high water demands from colonizing deserts from adjacent habitats or even dispersing across them to reach suitable habitats on the other side. Similarly, most aquatic organisms are unable to travel between freshwater and marine habitats, not because of physical barriers, but because of negative physiological responses to changes in salt content.
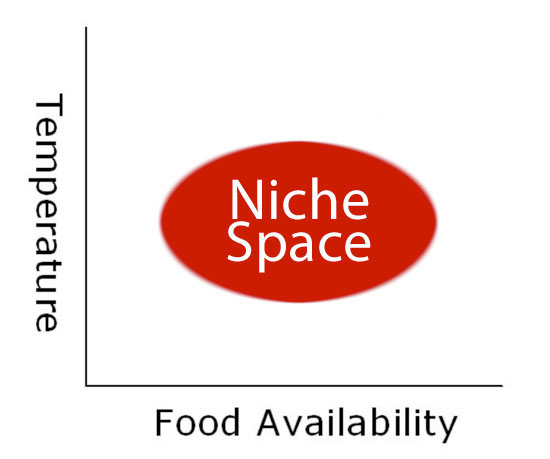
The particular range of conditions that species can tolerate, and how their physiological responses impact their geographic distributions, are commonly described in terms of their ecological niche (Hutchinson 1958), which essentially describes the position or function of a species within an ecosystem. Species survive within a range of values for any selected environmental variable, such as salinity or temperature. The multidimensional space encompassing suitable conditions for all factors is termed the niche space (Figure 1) and represents the conditions under which an individual, population, or species will persist. Within this niche space, the fundamental niche encompasses the total range of environmental conditions that are suitable for existence without the influence of such factors as competition and predation; the realized niche, however, is typically smaller and describes the fraction of the fundamental niche that is actually occupied by a species. As these relationships pertain to the distributions of species, spatial gradients in environmental factors often produce predictable patterns in which habitat quality is highest in the centers of species’ ranges and becomes more unsuitable as the range edge is approached. While the conditions at range edges may not be sufficient to impose mortality on individuals directly in many cases (Gaston 2003), changes in habitat quality at or near range boundaries serve as potent barriers to further range expansion through various non-lethal effects. Beyond the range limit, one or more environmental variables are beyond the species’ limits of tolerance (i.e., out of the niche space), making the habitat wholly unsuitable and preventing further dispersal and range expansion.

The evidence for climate impacts on distributional patterns is present in both fossil and living species. Among fossils species, patterns of migration coinciding with advancing glaciation and subsequent re-colonization following glacial retreat have been documented for many species (Graham et al. 1996). More rapid changes in spatial distribution are visible today in the face of global climate change, because the ranges of many species have shifted, either to higher altitudes in the case of montane species (Figure 2-3), or towards more northern latitudes in response to persistent warming trends occurring over very short time frames (Parmesan 2006).

Habitat, Individual, and Population Characteristics
Based on spatial gradients in habitat suitability, species often exhibit an abundant center distribution (Figure 4), wherein the highest population densities are observed in the range core, but the species becomes increasingly rare towards its range margin (Andrewartha & Birch 1954; Brown et al. 1995; Sagarin & Gaines 2002). The abundant center distribution can be exhibited as reduced numbers of populations at range edges, reduced densities within these peripheral populations, and even reduced fitness of individuals within peripheral populations. Those individuals occurring at range limits experience greater physiological stress due to suboptimal conditions, which influences such factors as dispersal, habitat selection, and subsequent reproductive fitness, or the number of offspring an individual produces that survive to reproduce.

At range edges, colonizable habitat may be abundant but of low overall quality, or higher quality habitat patches may be located further from one another relative to patches in the center of the range. According to optimal foraging theory, which relates the costs of moving among different habitat patches to habitat quality and the distances separating patches, individuals in peripheral populations may be unable to disperse to adjacent habitats due to the energetic costs involved. Such barriers prevent species from dispersing beyond current range limits, and may also prevent dispersal between core and peripheral populations. Without frequent immigration from core populations to replenish individuals lost in peripheral populations, the latter may exhibit an increased incidence of local extinction. Alternatively, peripheral populations may turn to colonizing habitats entirely different than those of core populations due to the unavailability of optimal habitats at range margins; however, such habitat switching is often associated with increased mortality.
In cases where the geographic distributions are characterized by reduced densities within peripheral populations, such patterns influence reproductive fitness and local adaptation, which further reduce the ability of species to expand beyond their current range limits. Due to the increased physiological stresses of inhabiting areas at or near range limits, individuals in peripheral populations may exhibit major differences in morphological and life history traits relative to individuals in core populations, such as smaller sizes, shorter life-spans, and reduced reproductive fitness. Reductions in reproductive fitness within peripheral populations may be due to individuals breeding less frequently, producing a smaller number of offspring per breeding event, or producing offspring with a reduced rate of survival relative to those in core populations. Also, as a consequence of reduced population densities and increased distances between habitat patches, individuals living at range margins may also experience difficulties in even finding a mate. The combined influences of isolation among habitat patches, reduced population density, and reduced reproductive fitness collectively may contribute to reduced genetic diversity among peripheral populations. The effects of reduced genetic diversity may include a greater likelihood of local extinction and inbreeding among marginal populations, as well as an inability to adapt to local conditions at the range edge and expand past current range limits (Howes & Lougheed 2008). However, recent studies have also concluded that some species actually exhibit increased genetic variation at range edges (Budd & Pandolfi 2010) facilitating range expansions, though such expansions may result in evolution of new species at range edges rather than simple range expansion within species (Malay & Paulay 2010).
Climatic factors and their influences on habitat suitability, population density, and subsequent reproductive fitness within species strongly reinforce range limits, and may serve to maintain abundant center distributions. However, the negative effects of unsuitable habitats at range margins on single species can be exacerbated by an assortment of interspecific interactions, including predation, competition, mutualism, and parasitism/disease.
Species Interactions
Predation
Competition
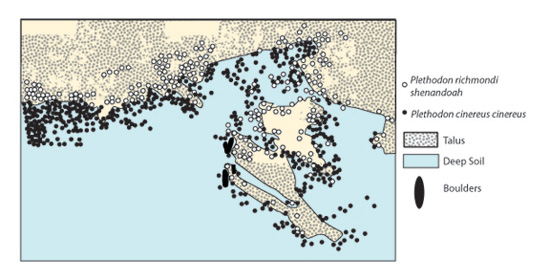
While few direct tests exist for determining the impact that interspecific competition alone may exert on the range limits of competing species, there is a great deal of evidence suggesting that competition at least partially contributes to geographic distributions. In many salamander species, for example, the ranges of ecologically similar species may overlap with one another, but at local scales within these areas of sympatry, these species are not found within the same habitat (Figure 5) despite their similar life history strategies. Subsequent investigations have found interspecific competition for food and suitable habitat between species in areas of sympatry to be quite intense, and often one species is forced into suboptimal habitat in the presence of a competitively superior species (Jaeger 1971a & b, 1972; Hairston 1987). Along these lines, individuals of a selected species occurring in sympatric zones with competing species often exhibit reduced fitness relative to individuals occurring outside sympatric zones, and these declines in fitness are often attributed to interspecific competition.
In some cases, the effects of climatic variables may increase the impact of competition between species on distributional patterns, or the outcome of competitive interactions may even be reversed by climatic influences. For example, natural fire cycles that remove woody plants typically facilitate the survival of fast-growing, fire-adapted grassland species. However, when fire is suppressed by human intervention or prolonged wet conditions, woody species that would otherwise be eliminated persist, and out-compete grasses (Brown & Lomolino 1998).
Mutualism
Parasitism/Disease
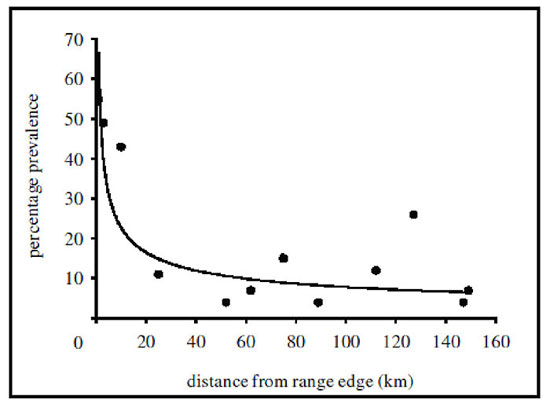
The degree to which parasites and disease may limit the geographic ranges of species has not been well-studied, although these relationships are thought to be important based on considerations of the abundant center distribution. For parasites that are specific to a single host species, it is intuitive that the parasite’s geographic distribution will be constrained to areas colonized by the host. At range edges, host individuals are more likely to be under greater physiological stress associated with the suboptimal habitat conditions described previously, which may make them more vulnerable to parasitic infection and/or disease (Figure 6). Under such conditions, the increased prevalence or per capita effect of parasites and disease among peripheral populations may contribute to an increased potential for the local extinction of host species, which could reinforce current boundaries to their geographic distribution. However, populations of many species also exhibit reduced densities at range margins, and in such areas it may be difficult for parasites or diseases to persist if hosts are not encountered often enough. In such cases, it may be that host population densities actually determine the geographic limits of their parasites, though more research is needed to address these issues.
Combined Influences
References and Recommended Reading
Andrewartha, H. G. & Birch, L. C. The Distribution and Abundance of Animals. Chicago, IL: University of Chicago Press, 1954.
Briers, R. A. Range limits and parasite prevalence in a freshwater snail. Proceedings of the Royal Society of London B 270, 178–180 (2003).
Brown, J. H. On the relationship between abundance and distribution of species. American Naturalist 124, 255–279 (1984).
Brown, J. H. & Lomolino, M. V. Biogeography. 2nd ed. Sunderland, MA: Sinauer & Associates, 1998.
Brown, J. H., Mehlman, D. W. et al. Spatial variation in abundance. Ecology 76, 2028–2043 (1995).
Budd, A. F. & Pandolfi, J. M. Evolutionary novelty is concentrated at the edge of coral species distributions. Science 328, 1558–1561 (2010).
Gaston, K. J. The Structure and Dynamics of Geographic Ranges. Oxford, UK: Oxford University Press, 2003.
Graham, R. W., Lundelius, Jr., E. L. et al. Spatial response of mammals to late quaternary environmental fluctuations. Science 272, 1601–1606 (1996).
Hairston, N. G. Community Ecology and Salamander Guilds. Cambridge, UK: Cambridge University Press, 1987.
Holt, R. D. & Barfield, M. Trophic interactions and range limits: the diverse roles of predation. Proceedings of the Royal Society B 276, 1435–1442 (2009).
Howes, B. J. & Lougheed, S. C. Genetic diversity across the range of a temperate lizard. Journal of Biogeography 35, 1269–1278 (2008).
Hutchinson, G. E. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22, 415–427 (1958).
Jaeger, R. G. Potential extinction through competition between two species of terrestrial salamanders. Evolution 24, 632–642 (1970).
Jaeger, R. G. Moisture as a factor influencing the distributions of two species of terrestrial salamanders. Oecologia 6, 191–207 (1971a).
Jaeger, R. G. Competitive exclusion as a factor influencing the distributions of two species of terrestrial salamanders. Ecology 52, 632–637 (1971b).
Jaeger, R. G. Food as a limited resource in competition between two species of terrestrial salamanders. Ecology 53, 535–546 (1972).
Lawler, J. J., Shafer, S. L. et al. Projected climate impacts for the amphibians of the western hemisphere. Conservation Biology 24, 38–50 (2010).
Malay, M. C. D. & Paulay, G. Peripatric speciation drives diversification and distributional pattern of reef hermit crabs (Decapoda: Diogenidae: Calcinus). Evolution 64, 634–662 (2010).
Parmesan, C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology and Systematics 37, 637–669 (2006).
Sagarin, R. & Gaines, S. The "abundant centre" distribution: to what extent is it a biogeographical rule? Ecology Letters 5, 137–147 (2002).
Savidge, J. A. Extinction of an island forest avifauna by an introduced snake. Ecology 68, 660–668 (1987).






























