« Prev Next »

The laws of physics describe the interactions between energy and mass: the energy in a closed system is conserved, and matter can neither be created nor destroyed. Modern physics has shown that reality is more complex at very large and very small scales (e.g., Einstein’s famous equation, E = mc2 demonstrated that mass can be converted to energy in the sun or nuclear reactors), but in the context of Earth’s ecosystems, energy is conserved and matter can neither be created nor destroyed. This seemingly simplistic statement has profound consequences when we study how ecosystems function. In particular, the energy present within an ecosystem is collected and shared by organisms in many different ways; this "sharing" takes place through ecological interactions, such as predator-prey dynamics and symbioses. When we move to the ecosystem level, however, we consider interactions among organisms, populations, communities, and their physical and chemical environment. These interactions have an important bearing on the structure of organisms, ecosystems, and, over geologic time, the planet itself.
A primary example of this involves the energy currency in ecosystems, which is carbon. The amount and form of carbon present in different ecosystem pools — such as plants, animals, air, soil, and water — is controlled by organisms, and ultimately affects their ecological success. How much carbon dioxide (CO2) is present in the atmosphere is a major regulator of Earth’s climate; until humans began burning fossil fuels in large quantities, over timescales from years to centuries, CO2 concentrations were controlled entirely by plants and microorganisms (with small contributions from animals, and periodically, important geological sources like volcanoes). How does this exchange of carbon among organisms and inorganic pools within ecosystems work? The answer has two important parts to it: the first has to do with how energy is generated in ecosystems, and the second involves how energy is used.
Energy Production
There are three important exceptions to this, one that is significant in ecosystems, and two that have little influence in ecology. Both geothermal heat and nuclear energy have been harnessed by humans, but are not used by other organisms; these may be important energy sources in our cities, but not in natural ecosystems. In contrast, many microorganisms can generate energy by performing chemical reactions that convert compounds to different chemical forms, and release energy in the process. These are known as oxidation-reduction (or redox) reactions. Some of these microbes can use chemical energy to fix CO2 — just like photosynthetic organisms, but using chemical redox reactions rather than sunlight. This is not an important process at the global scale, but it can be very important in certain circumstances. For example, nitrification is an essential biogeochemical process that is carried out by these "chemoautotrophs" globally. Among the best known examples are the remarkable ecosystems that flourish at deep-sea vents. Vent communities are not supported by the heat coming out of these vents, but are instead sustained by the chemical compounds (e.g., methane and hydrogen sulphide) that are discharged from the vents and chemically converted by microorganisms, thereby generating energy. This chemical energy is the major source of energy supporting these ecosystems in their entirety, and before photosynthesis evolved, all of life on Earth was sustained in similar ways by the use of chemical energy.
Energy Consumption
Photosynthesis: 6CO2 + 6H2O + light energy → C6H12O6 (glucose) + 6O2
Respiration: C6H12O6 + 6O2 → 6CO2 + 6H2O + chemical energy
Because many different organisms (from bacteria to animals) can make use of the material that photoautotrophs produce, almost all of it gets used, and photosynthesis and respiration are often closely balanced over time. This leads to several key insights. First, CO2 that is fixed by photoautotrophs is eventually returned to the atmosphere; this may not be exactly equal — particularly as a result of human modification of ecosystems, and also over geological time scales — but it is usually close to equal. Second, this is balanced by nearly equal release and consumption of oxygen, and consumption and release of water. Because these production and consumption terms tend to balance one another, they cancel out if we combine the equations above: the CO2 and water that are consumed by photosynthesis are regenerated by respiration; the oxygen produced by photosynthesis is consumed by respiration. The net effect of photosynthesis and respiration is for light energy to be converted to chemical energy. This is the conservation of energy in ecosystems: the sunlight absorbed by plants and microbes ultimately goes on to power the entire ecosystem.
Conservation of Energy: Balancing the Budget
Carbon acts as an energy currency in ecosystems because light is converted to organic carbon compounds (sugars, fats, proteins), and organic carbon compounds are then converted to chemical energy. Hence, the total amount of organic material that is produced by plants is directly related to the amount of light energy that is absorbed. This is primary production, a term that refers to the growth of plants or change in their total biomass. It is referred to as ‘primary’ production because it is biomass that is produced directly from CO2, whereas secondary production is produced from already existing organic material. Primary production is expressed in terms of carbon fixed per unit time and per unit space, and represents a fundamental property of an ecosystem, which is the rate of energy generated over time.
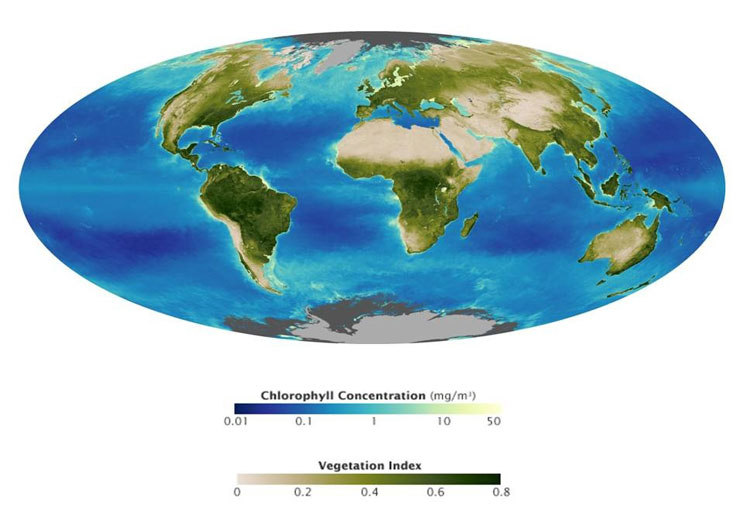
As one might imagine, measuring all plant growth in a forest or all phytoplankton growth in a sea is extremely challenging. However, because this is ultimately related to the amount of light energy that is absorbed, and we can measure absorbed and emitted light using satellites in space, scientists have figured out clever ways to measure primary production using satellite data. This works because ecosystems seem to have a relatively consistent light use efficiency (LUE) that represents the proportion of light absorbed that is converted to biomass. Interestingly, this is a small proportion of the total energy that reaches Earth from the sun — a lot of sunlight is reflected, or absorbed and re-radiated back to space — and if you look around and see how much plant growth or primary production occurs, you get an idea of how much energy the sun can provide. Satellite data have given us broad pictures of the living biosphere that can be used to determine the total amount of production, carbon, and energy that cycles through ecosystems (Figure 1).

How primary production, energy, and carbon are subsequently used varies even more widely from ecosystem to ecosystem. Much of it is consumed by herbivores, but some of it falls to the ground or to the deep ocean bottom; some of what herbivores eat becomes biomass, but much is respired, and some becomes waste. These relative proportions vary spatially and temporally, but eventually, carbon produced by plants is used by the diversity of organisms present in a given ecosystem. Even ‘waste’ products are a rich source of energy and other nutrients for microorganisms such as bacteria and fungi, and so waste is consumed over time. Some carbon remains resistant to attack, or ‘recalcitrant,’ (for example, many organic compounds in soil are recalcitrant), but most fixed carbon can be used as a source of energy, and is then returned to the atmosphere as CO2. All these carbon fluxes are often partitioned into an alphabet soup of gross primary production (GPP), net primary production (NPP), plant respiration (Rplant), heterotroph respiration (Rheterotroph), net ecosystem production (NEP), and net ecosystem exchange (NEE); these are concepts that are explored in ecosystem ecology and exhibit remarkable patterns. For example, NPP, plant respiration, and heterotroph respiration all seem to be approximately equal across many ecosystems (Figure 2).
But what happens if this carbon budget becomes unbalanced? What would happen over time, for example, if heterotrophic respiration were a little less than NPP? The result is ecosystems such as peatlands, where organic material builds up in the soils. When those peatlands are drained and used in other ways (e.g. as oil palm plantations in southeast Asia), that carbon ends up back in the atmosphere. There are other important and interesting exceptions to the rule of carbon balance, and as humans continue to produce energy and food, we continue to alter the carbon balance of ecosystems.
Summary
In the end, because energy is conserved in ecosystems, the chemical energy used by buffalo and bacteria must be less than the chemical energy generated via primary production, because of unavoidable inefficiencies in energy capture. Photosynthesis is the dominant source of energy in most ecosystems through conversion of light to C-H bonds in organic material, and so the flow and fate or carbon is tightly linked to energy flow. Understanding how carbon and energy flow are linked to other elements and nutrients, support patterns of biodiversity, and connect carnivores, herbivores, and plants, will all draw on this fundamental fact.
References and Recommended Reading
Chapin, F. S., Matson, P. A. et al. Principles of Terrestrial Ecosystem Ecology. New York, NY: Springer, 2002.
del Giorgio, P. A. & Duarte, C. M. Respiration in the open ocean. Nature 420, 379-384 (2002).
Field, C. B., Behrenfeld, M. J. et al. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 281, 237-240 (1998).
Harte, J., & Kinzig, A. P. Mutualism and Competition between Plants and Decomposers: Implications for Nutrient Allocation in Ecosystems. Am Nat 141, 829 (1993).
Raich, J. W. & Schlesinger, W. H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44, 81-99 (1992).
Raven, J. A. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat Microb Ecol 56, 177-192 (2009).






























