« Prev Next »

Atmospheric concentrations of carbon dioxide have been steadily rising, from approximately 315 ppm (parts per million) in 1959 to a current atmospheric average of approximately 385 ppm (Keeling et al.,2009). Current projections are for concentrations to continue to rise to as much as 500–1000 ppm by the year 2100 (IPCC 2007).
While a great deal of media and public attention has focused on the effects that such higher concentrations of CO2 are likely to have on global climate, rising CO2 concentrations are also likely to have profound direct effects on the growth, physiology, and chemistry of plants, independent of any effects on climate (Ziska 2008). These effects result from the central importance of CO2 to plant metabolism. As photosynthetic organisms, plants take up atmospheric CO2, chemically reducing the carbon. This represents not only an acquisition of stored chemical energy for the plant, but also provides the carbon skeletons for the organic molecules that make up a plants’ structure. Overall, the carbon, hydrogen and oxygen assimilated into organic molecules by photosynthesis make up ~96% of the total dry mass of a typical plant (Marschner 1995). Photosynthesis is therefore at the heart of the nutritional metabolism of plants, and increasing the availability of CO2 for photosynthesis can have profound effects on plant growth and many aspects of plant physiology.
Our knowledge of plant responses to future CO2 concentrations rests on the results of experiments that have experimentally increased CO2 and then compared the performance of the experimental plants with those grown under current ambient CO2 conditions. Such experiments have been performed in a wide variety of settings, including greenhouses and chambers of a variety of sizes and designs. However plants grown in chambers may not experience the effects of increasing CO2 the same way as plants growing in more natural settings. For this reason, techniques of Free-Air Carbon dioxide Enrichment (FACE) have been developed that allow natural or agricultural ecosystems to be fumigated with elevated concentrations of CO2 in the field without use of chambers (Figure 1). As these experiments are the most naturalistic, they should provide the best indication of the responses of plants to increased CO2 under the real-world conditions of the future. This article therefore focuses on data from FACE experiments wherever these are available. Whenever possible, to ensure the generality of conclusions, reference is made to analyses that have incorporated data from multiple experiments independently conducted at various research facilities.

One of the most consistent effects of elevated atmospheric CO2 on plants is an increase in the rate of photosynthetic carbon fixation by leaves. Across a range of FACE experiments, with a variety of plant species, growth of plants at elevated CO2 concentrations of 475–600 ppm increases leaf photosynthetic rates by an average of 40% (Ainsworth & Rogers 2007). Carbon dioxide concentrations are also important in regulating the openness of stomata, pores through which plants exchange gasses, with the external environment. Open stomata allow CO2 to diffuse into leaves for photosynthesis, but also provide a pathway for water to diffuse out of leaves. Plants therefore regulate the degree of stomatal opening (related to a measure known as stomatal conductance) as a compromise between the goals of maintaining high rates of photosynthesis and low rates of water loss. As CO2 concentrations increase, plants can maintain high photosynthetic rates with relatively low stomatal conductance. Across a variety of FACE experiments, growth under elevated CO2 decreases stomatal conductance of water by an average of 22% (Ainsworth & Rogers 2007). This would be expected to decrease overall plant water use, although the magnitude of the overall effect of CO2 will depend on how it affects other determinants of plant water use, such as plant size, morphology, and leaf temperature. Overall, FACE experiments show decreases in whole plant water use of 5–20% under elevated CO2. This in turn can have consequences for the hydrological cycle of entire ecosystems, with soil moisture levels and runoff both increasing under elevated CO2 (Leakey et al. 2009).
Since photosynthesis and stomatal behavior are central to plant carbon and water metabolism, growth of plants under elevated CO2 leads to a large variety of secondary effects on plant physiology. The availability of additional photosynthate enables most plants to grow faster under elevated CO2, with dry matter production in FACE experiments being increased on average by 17% for the aboveground, and more than 30% for the belowground, portions of plants (Ainsworth & Long 2005; de Graaff et al. 2006). This increased growth is also reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments (Ainsworth 2008; Long et al. 2006).
Elevated CO2 also leads to changes in the chemical composition of plant tissues. Due to increased photosynthetic activity, leaf nonstructural carbohydrates (sugars and starches) per unit leaf area increase on average by 30–40% under FACE elevated CO2 (Ainsworth 2008; Ainsworth & Long 2005). Leaf nitrogen concentrations in plant tissues typically decrease in FACE under elevated CO2, with nitrogen per unit leaf mass decreasing on average by 13% (Ainsworth & Long 2005). This decrease in tissue nitrogen is likely due to several factors: dilution of nitrogen from increased carbohydrate concentrations; decreased uptake of minerals from the soil, as stomatal conductance decreases and plants take up less water (Taub & Wang 2008); and decreases in the rate of assimilation of nitrate into organic compounds (Bloom et al. 2010).
Protein concentrations in plant tissues are closely tied to plant nitrogen status. Changes in plant tissue nitrogen are therefore likely to have important effects on species at higher trophic levels. Performance is typically diminished for insect herbivores feeding on plants grown in elevated CO2 (Zvereva & Kozlov 2006). This can lead to increased consumption of plant tissues as herbivores compensate for decreased food quality (Stiling and Cornelissen 2007). Effects on human nutrition are likely as well. In FACE experiments, protein concentrations in grains of wheat, rice and barley, and in potato tubers, are decreased by 5–14% under elevated CO2 (Taub et al. 2008). Crop concentrations of nutritionally important minerals including calcium, magnesium and phosphorus may also be decreased under elevated CO2 (Loladze 2002; Taub & Wang 2008).
Effects of Other Environmental Factors on Plant Response to Elevated CO2
Another environmental factor that interacts with elevated CO2 is atmospheric ozone (O3), a gaseous toxin. Ground-level O3 concentrations have been increasing worldwide (and are expected to continue to increase) due to increased emissions of pollutants that react to produce O3 (Vingarzan 2004). High atmospheric concentrations of ozone can cause damage to leaves and decreased plant growth and photosynthesis (Feng et al. 2008; Morgan et al. 2003). The primary location of O3 injury to plants is the internal tissues of leaves. Decreased openness of stomata under elevated CO2 can therefore decrease exposure of sensitive tissues to ozone. Elevated CO2 substantially decreases the negative effects of high ozone on photosynthesis, growth, and seed yield in both soybeans and rice (Feng et al. 2008; Morgan et al. 2003). Across experiments with all plant species, the enhancement of growth by elevated CO2 is much greater under conditions of ozone stress than otherwise (Poorter & Navas 2003).
Differences among Plant Functional Types in Response to Rlevated CO2
The preceding discussion has presented the average effects of elevated CO2, but obscures important patterns of difference in response among plant species. One of the most important determinants of species differences in response to elevated CO2 is photosynthetic type. Most plant species (~90%) utilize a photosynthetic process known as C3 photosynthesis. Other species use either of two physiologically distinct processes known as C4 and CAM photosynthesis (Figure 2). C4 plants include most tropical and sub-tropical grasses and several important crops, including maize (corn), sugar cane, sorghum, and the millets. There has therefore been considerably more research on the responses to elevated CO2 in C4 than in CAM plants.
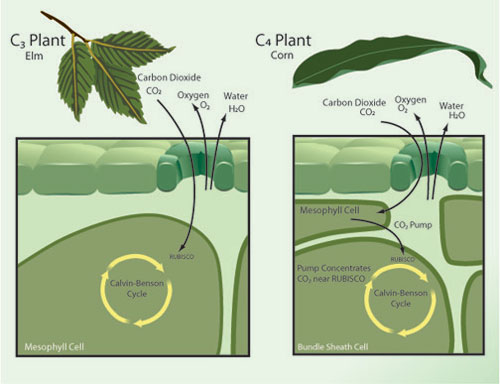
C4 plants use a biochemical pump to concentrate CO2 at the locations within the leaf where the RUBISCO enzyme mediates incorporation of CO2 by the Calvin-Benson photosynthetic cycle. Since CO2 concentrations are already high within the bundle sheath cells, increasing atmospheric CO2 concentrations above current levels has little direct effect on photosynthetic rates for C4 species. C4 species do respond to elevated CO2 by decreasing stomatal conductance; this may lead to some indirect enhancement of photosynthesis by helping avoid water stress under drought conditions (Leakey 2009). In FACE experiments, stimulation of photosynthesis by elevated CO2 in C4 plants is only about one-third of that experienced by C3 species. C4 plants also show little or no enhancement of growth (dry matter production) in these studies (Ainsworth & Long 2005). The very limited data available also shows no increase in C4 crop yield in FACE studies (Long et al. 2006). While there is little FACE data available on effects of elevated CO2 on plant nitrogen and protein concentrations, data from chamber experiments shows C4 plants to be much less responsive than C3 plants in this regard (Cotrufo et al. 1998). The picture that emerges is that C4 plants are in general relatively unresponsive to elevation of atmospheric CO2 above current ambient levels.
In contrast to C4 species, another group of plants, legumes (members of the botanical family Fabaceae) may be especially capable of responding to elevated CO2 with increased photosynthesis and growth (Rogers et al. 2009). For most plants, growth under elevated CO2 can alter the internal balance between carbon (obtained in extra quantities through enhanced photosynthesis) and nitrogen (either unaffected or taken up in decreased amounts due to decreased uptake of water). In contrast, most legume species participate in close mutualistic relationships with bacteria that live in nodules formed on the plant’s roots. These bacteria are able to "fix" atmospheric nitrogen, chemically reducing it to a form that can be taken up and used by plants. Under elevated CO2 conditions, legumes may be able to shunt excess carbon to root nodules where it can serve as a carbon and energy source for the bacterial symbionts. In effect, legumes may be able to exchange the excess carbon for nitrogen and thereby maximize the benefits of elevated atmospheric CO2. Many studies in controlled environments have shown that, compared to other plant species, legumes show greater enhancement of photosynthesis and growth by elevated CO2 (Rogers et al. 2009). Decreases in tissue nitrogen concentrations under elevated CO2 are also smaller for legumes than for other C3 species (Cotrufo et al. 1988; Jablonski et al. 2002; Taub et al. 2008). In FACE experiments, soybeans (a legume) show a greater response to elevated CO2 than wheat and rice in photosynthesis and overall growth, although not in harvestable yield (Long et al. 2006).
Plant Community Interactions under Elevated CO2
Summary
Current evidence suggests that that the concentrations of atmospheric CO2 predicted for the year 2100 will have major implications for plant physiology and growth. Under elevated CO2 most plant species show higher rates of photosynthesis, increased growth, decreased water use and lowered tissue concentrations of nitrogen and protein. Rising CO2 over the next century is likely to affect both agricultural production and food quality. The effects of elevated CO2 are not uniform; some species, particularly those that utilize the C4 variant of photosynthesis, show less of a response to elevated CO2 than do other types of plants. Rising CO2 is therefore likely to have complex effects on the growth and composition of natural plant communities.
References and Recommended Reading
Ainsworth, E. A. Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biology 14, 1642-1650 (2008).
Ainsworth, E. A. & Long, S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351-372 (2005).
Ainsworth, E. A. & Rogers, A. The response of photosynthesis and stomatal conductance to rising (CO2): mechanisms and environmental interactions. Plant, Cell and Environment 30, 258-270 (2007).
Bloom, A. J., Burger, M. et al. Carbon dioxide inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899-903 (2010).
Cotrufo, M. F., Ineson, P. et al. Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biology 4, 43-54 (1998).
Feng, Z., Kobayashi, K. et al. Impact of elevated ozone concentration on growth, physiology and yield of wheat (Triticum aestivum L.): a meta-analysis. Global Change Biology 14, 2696-2708 (2008).
de Graaff, M. A., Van Groenigen, K. J. et al. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biology 12, 2077-2091 (2006).
IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press, 2007.
Jablonski, L. M., Wang, X. et al. Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytologist 156, 9-26 (2002).
Keeling, R. F., Piper, S. C. et al. Atmospheric CO2 records from sites in the SIO air sampling network. In Trends: A Compendium of Data on Global Change (Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, 2009).
Leakey, A. D. B., Ainsworth, E. A. et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations; six important lessons from FACE. Journal of Experimental Botany 60, 2859-2876 (2009).
Loladze, I. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends in Ecology & Evolution 17, 457-461 (2002).
Long, S. P., Ainsworth, E. A. et al. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918-1921 (2006).
Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed. London, UK: Academic Press, 1995.
Morgan, P.B., Ainsworth, E.A. et al. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant, Cell and Environment 26, 1317-1328 (2003).
Poorter, H. and Navas, M. L. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist 157, 175-198 (2003).
Rogers, A., Ainsworth, E. et al. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiology 151, 1009-1016 (2009).
Stiling, P. & Cornelissen, T. How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Global Change Biology 13, 1823-1842 (2007).
Taub, D., Miller, B. et al. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biology 14, 565-575 (2008).
Taub, D. R. & Wang, X. Z. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology 50, 1365-1374 (2008).
Vingarzan, R. A review of surface ozone background levels and trends. Atmospheric Environment 38, 3431-3442 (2004).
Ziska, L. H. Rising atmospheric carbon dioxide and plant biology: the overlooked paradigm. In Controversies in Science and Technology, From Climate to Chromosomes. eds. Kleinman, D.L., Cloud-Hansen, K.A. et al. (New Rochele: Liebert, Inc. 2008) 379-400.
Zvereva, E. L. & Kozlov, M. V. Consequences of simultaneous elevation of carbon dioxide and temperature for plant






























