« Prev Next »
The majority of pathogens of animals are generalists that infect multiple host species, referred to as multi-host pathogens or multi-host parasites. Some multi-host pathogens are maintained in a sylvatic transmission cycle where the pathogen is maintained completely in multiple wildlife species. Among domesticated animal species, roughly 77% of pathogens of livestock and 90% of pathogens of domestic carnivores are known to be multi-host pathogens (Cleaveland et al. 2001). Over 60% of all known human pathogens are zoonotic (Taylor et al. 2001), meaning they originate in animals but can cross-infect humans. In some cases, humans can go on to infect humans or other animals (e.g., plague), while in others (e.g., West Nile Virus) humans are dead-end hosts. In the latter case, the pathogen causes disease in an individual human but further transmission to other hosts or vectors does not occur.
Why and how do pathogens infect multiple host species?
The ability to infect multiple host species is not limited to pathogens of a specific type (e.g., virus, bacteria, helminth) or pathogens employing a particular mode of transmission (Figure 1). The following are only a small subset of multi-host pathogens (or diseases caused by multi-host pathogens) listed by their mode of transmission:
- Close contact/direct transmission (including direct contact, airborne, aerosol, bite, or sexual transmission): SARS, rabies, monkeypox, influenza, hantavirus, herpes, SIV (simian immunodeficiency virus)
- Non-close/indirect transmission (including fomites, environmental transmission): cholera, avian influenza, anthrax, brucellosis
- Intermediate host: Schistosomiasis, Dicrocoelium dendriticum
- Vector-borne transmission: West Nile virus, Lyme disease, Chikungunya.
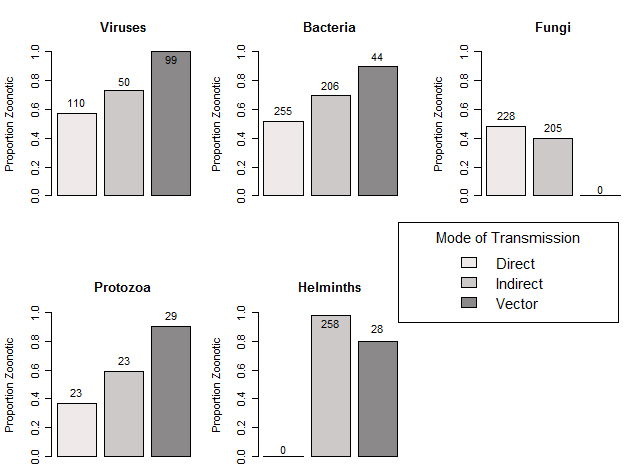
Depending on the mode of transmission, some pathogens are considered to be obligate multi-host pathogens or parasites; these include parasites with complex life cycles and vector-borne pathogens. Parasites that exhibit a complex life cycle require a definitive host for reproduction and one or more intermediate host species for growth and development. Vector-borne pathogens are transmitted between hosts by an intermediate organism, often an arthropod like mosquitoes or ticks, referred to as a vector.
Several factors can enable a pathogen to infect multiple host species (e.g., Pulliam & Dushoff 2009). For example, genetic change in the pathogen can occur through selection or through random mutations, allowing the pathogen to become better adapted to infect a new host species (Pepin et al. 2010). It is generally believed that the higher a pathogen's mutation rate, the more genetically diverse it will be and therefore the more likely it is that the pathogen is a generalist. For example, RNA viruses mutate about 300 times faster than DNA viruses, and directly transmitted RNA viruses of humans are more likely to be zoonotic than directly transmitted DNA viruses of humans (Drake 1993, Woolhouse et al. 2001). Host speciation is another mechanism by which a pathogen that originally infects an ancestral host comes to infect multiple new host species (e.g., Garamszegi 2009). Generally, pathogens tend to infect host species that are phylogenetically similar to each other because these host species share traits (e.g., immunologic, antigenic, or ecological similarities) that make them susceptible to the same pathogens. Conversely, more distantly related host species do not share as many traits, decreasing the chances that they will share pathogen species (Freeland 1983, Davies & Pedersen 2008). Recently speciated hosts share genetic similarities, potentially allowing a pathogen to infect both species. Introductions of non-native hosts and pathogens can also result in the infection of a new host species by providing new opportunities for infectious contact between pathogens and naïve hosts (e.g., Peeler et al. 2011).
Infecting a wide range of host species is one way in which a pathogen's chance of persistence is increased. The ability to infect multiple host species is not always adaptive, however, and several ecological trade-offs are associated with the benefit of a broad host range. For example, while single-host pathogens tend to evolve an intermediate level of virulence in their host, virulence evolution in multi-host pathogens is more complex. A multi-host pathogen could be highly virulent in one host while exhibiting low virulence in another. The optimal virulence in each host will depend on how each host contributes to pathogen fitness (Regoes et al. 2000, Gandon 2004, Rigaud et al. 2010). Another cost of infecting multiple host species is the degree to which a pathogen can adapt to a host's immune system. If a pathogen only infects one host species, the pathogen can evolve to become highly proficient at evading the immune system of that host. In multi-host pathogens, however, an adaptation in one host species may be maladaptive in another host species (Elena et al. 2009). For example, many vector-borne pathogens are viruses, and thus are expected to have a great deal of genetic diversity due to high mutation rates (Cooper & Scott 2001, Ciota et al. 2007). However, experimental research (i.e., serial passage experiments involving transmission between an invertebrate vector and a vertebrate host) has shown that viral genetic sequences are largely unchanged after multiple transmissions between very different species. Moreover, viruses that were experimentally allowed to transmit between members of only one species rapidly adapted to that species, with coinciding loss of fitness often observed in the bypassed species (Romanova et al. 2007, Coffey et al. 2008, Vasilakis et al. 2009). This host alternation is, therefore, a potential constraint on the genetic diversity of multi-host pathogens.
Invasion and population dynamics of multi-host pathogens
The invasion of a naïve population of hosts and subsequent epidemiological dynamics of multi-host pathogens are inherently different from single host systems because multiple host species provide multiple invasion pathways as well as multiple transmission routes. That is, if infection is unsuccessful in one host species, the presence of another host species provides an alternative route for the pathogen to invade a community. Both invasion and persistence are related to a theoretical quantity, R0, referred to as the basic reproductive number and defined as the number of secondary infections resulting from a single primary infection in a completely susceptible population. If R0>1, then an introduced pathogen is likely to persist and may cause an epidemic in the host population (Anderson & May 1991). In a community comprised of multiple host species, R0 may be greater than one for one species, but less than one for another species. In this case, the community composition would determine whether or not the pathogen will persist at the community level. The form of transmission (i.e., density-dependent versus frequency-dependent) also has implications for population dynamics of multi-host pathogens (Dobson 2004). Single-host pathogens that rely on density-dependent transmission rarely drive their host to extinction because the host population will drop below a threshold size such that pathogen transmission can no longer be maintained (Grenfell & Dobson 1995, Hudson et al. 2002). Infecting multiple host species, as well as exhibiting frequency-dependent transmission (e.g., sexually transmitted or vector-borne pathogens), increases the chance of pathogen-induced host extinction because the threshold density for pathogen persistence is eliminated (de Castro & Bolker 2005).
In host-pathogen systems with multiple hosts, disease dynamics can also depend on the competence of each host species for harboring and transmitting the pathogen, as well as the relative frequency of transmission between host and vector species (LoGuidice et al. 2003). Accordingly, the community composition of potential hosts can have a large effect on pathogen dynamics, especially when competence within the host community varies substantially (Holt et al. 2003, LoGuidice et al. 2008). Specifically, theory suggests that, in multi-host vector-borne pathogen systems, more diverse host communities may reduce pathogen transmission by decreasing contacts between infected vectors and highly competent hosts compared with single-species host systems (Schmidt & Ostfeld 2001, Keesing et al. 2006). This phenomenon, referred to as the dilution effect, has been studied primarily in the Lyme disease system of the Northeast U.S., but has also been demonstrated in other multi-host pathogen systems (Figure 2; Swaddle & Calos 2008, Dizney & Ruedas 2009, Hall et al. 2009). Some scientists have argued that while empirical evidence exists for the dilution effect in several multi-host pathogen systems, the mechanism by which disease dilution is occurring is often unknown (e.g., dilution versus density effects; Begon 2008).

Multi-host pathogens in a changing climate
Why should we study multi-host pathogens?
Multi-host pathogen systems are intrinsically complex, shaped by pathogen and host dynamics as well as evolutionary, environmental, and climatic interactions. Understanding multi-host pathogens from an ecological perspective provides a variety of potential applications. Multi-host pathogens can, for instance, affect organisms and ecological dynamics far outside their host range. Depending on their effect on a host species (e.g., high virulence/mortality, behavioral modification, reduced fitness/reproduction), multi-host pathogens may regulate not only populations and communities of host species, but also predator, prey, or competitor populations (Hatcher et al. 2006). Understanding the ecology and evolution of multi-host pathogens may also be important for species conservation and biodiversity preservation (McCallum & Dobson 1995, Smith et al. 2006). Some species that are now declining due at least in part to multi-host pathogens include bird species infected by avian malaria in Hawaii (Van Riper et al. 1986) and West Nile Virus in the continental U.S. (LaDeau et al. 2007), bat species in the U.S. infected with the pathogenic fungus (Geomyces destructans) that causes White-Nose Syndrome (Frick et al. 2010), and seals infected with phocine distemper virus in Europe (Swinton et al. 1998, Jensen et al. 2002). Understanding the ecology of multi-host pathogens, particularly zoonotic multi-host pathogens, can provide information needed for shaping human health policy and may contribute to outbreak detection and other warning systems, or be central to programs aimed at preventing or reducing transmission and human infections by multi-host pathogens.
Glossary
multi-host pathogen: A pathogen that infects multiple host species
sylvatic transmission: Transmission cycle of a pathogen maintained completely in non-human animals
zoonotic: Referring to a pathogen that infects humans, but originates from a non-human animal species
dead-end host: A host in which a pathogen can cause disease, but not maintain transmission
phylogenetic relatedness: Evolutionary distance among species; organisms that share a recent common ancestor and typically have genetic similarities
host range: The set of host species that a pathogen or parasite can infect, described by both the number of host species and the phylogenetic relatedness between host species.
direct transmission: Occurring from direct or close contact with infectious individuals, including aerosol/airborne transmission, sexual transmission, and transmission via a bite
indirect transmission: Occurring from non-close contact with infectious individuals, including fomites and environmental transmission
complex life cycle: A parasite life cycle that requires a definitive host for reproduction and one or more intermediate hosts for growth and development
vector: Organisms (primarily arthropods like mosquitoes, ticks, and fleas) that transmit a pathogen between host species
virulence: Pathogen-induced mortality or other decline in the fitness of a host caused by infection
fitness: The potential for an organism to survive and reproduce
host alternation: Pathogen transmission between two or more (often disparate) host species, which constrains pathogen adaptation to one host species over another
R0: The basic reproductive number; the number of secondary infections arising from an initial infection in a completely susceptible population
community composition: The number and relative abundance of host species in a community
density-dependent transmission: Transmission rate that increases with host density
density-independent transmission: Transmission rate that functions independent of host density
competence: The differential ability of an organism to harbor and transmit a pathogen
dilution effect: A net reduction in pathogen transmission from increasing host species diversity
References and Recommended Reading
Altizer S. et al. Animal migration and infectious disease risk. Science 331(6015): 296-302 (2011).
Anderson R.M. & R.M. May (Eds.). Population biology of infectious diseases. New York: Springer-Verlag(1982).
Begon M. Effects of host diversity on disease dynamics. In Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Eds. Ostfeld R.S.et al. Princeton, New Jersey: Princeton University Press, (2008).
Bosch J. et al. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc R Soc B 274(1607): 253-260 (2007).
Ciota A.T. et al. Cell-specific adaptation of two flaviviruses following serial passage in mosquito cell culture. Virology 357(2): 165-174 (2007).
Cleaveland S. et al. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Phil Trans R Soc Lond B 356(1411): 991-999 (2001).
Coffey L.L.. Arbovirus evolution in vivo is constrained by host alternation. PNAS 105(19): 6970-6975 (2008).
Cooper L.A. et al. Differential evolution of Eastern Equine Encephalitis virus populations in response to host cell type. Genetics 157(4): 1403-1412 (2001).
Davies T.J. & A.B. Pedersen. Phylogeny and geography predict pathogen community similarity in wild primates and humans. Proc R Soc B 275(1643): 1695-1701 (2008).
De Castro F. & B.Bolker.Mechanisms of disease-induced extinction. Ecology Letters 8(1): 117-126 (2005).
Dizney L.J. &L.A. Ruedas. Increased host species diversity and decreased prevalence of Sin Nombre virus. Emerg Infect Dis 15(7): 1012-1018 (2009).
Dobson A. Population dynamics of pathogens with multiple host species. Am Nat 164(S5): S64-S78 (2004).
Drake J.W.Rates of spontaneous mutation among RNA viruses. PNAS 90(9): 4171-4175 (1993).
Elena S.F. et al. The evolution of viruses in multi-host fitness landscapes. Open Virol J 3: 1-6 (2009).
Freeland W.J. Parasites and the coexistence of animal host species. Am Nat 121(2): 223-236 (1983).
Frick W.F. et al. An emerging disease causes regional population collapse of a common North American bat species. Science 329(5992): 679-682 (2010).
Gandon S. Evolution of multi-host parasites. Evolution 58(3): 455-469 (2004).
Garamszegi L.Z. Patterns of co-speciation and host switching in primate malaria parasites. Malaria Journal 8: 110 (2009).
Grenfell B.T. & A.P.Dobson (Eds). Ecology of infectious diseases in natural populations. Cambridge: Cambridge University Press(1995).
Hall S.R. et al. Friendly competition: evidence for a dilution effect among competitors in a planktonic host-parasite system. Ecology 90(3): 791-801 (2009).
Harvell C.D. et al. Climate warming and disease risks for terrestrial and marine biota. Science 296(5576): 2158-2162 (2002).
Harvell D. et al. Climate change and wildlife diseases: when does the host matter the most? Ecology 90(4): 912-920 (2009).
Hatcher M.J. et al.. How parasites affect interactions between competitors and predators. Ecology Letters 9(11): 1253-1271 (2006).
Holt R.D. et al. Parasite establishment in host communities. Ecology Letters 6(9): 837-842 (2003).
Hudson P.J. et al. (Eds). The ecology of wildlife diseases. Oxford: Oxford University Press ( 2002).
Jensen T. et al. Another phocine distemper outbreak in Europe. Science 297 (2002).Keesing F. et al. Effects of species diversity on disease risk. Ecology Letters 9(4): 485-498 (2006).
LaDeau S.L. et al West Nile virus emergence and large-scale declines of North American bird populations. Nature 447: 710-713 (2007).
Lafferty K.D. The ecology of climate change and infectious diseases. Ecology 90(4): 888-900 (2009).
LoGiudice K. et al. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. PNAS 100(2): 567-371 (2003).
LoGiudice K. et al. Impact of host community composition on Lyme disease risk. Ecology 89(10): 2841-2849 (2008).
Malpica J.M. et al. Association and host selectivity in multi-host pathogens. PLoS ONE 1(1): e41 (2006).
McCallum H. & A. Dobson. Detecting disease and parasite threats to endangered species and ecosystems. TREE 10(5): 190-194 (1995).
Pedersen A.B.Patterns of host specificity and transmission among parasites of wild primates. International Journal for Parasitology 35(6): 647-657 (2005).
Peeler E.J. et al. Non-native aquatic animal introductions have driven disease emergence in Europe. Biological Invasions 13(6): 1291-1303 (2001).
Pepin K.M. et al. Identifying genetic markers of adaptation for surveillance of viral host jumps. Nature Reviews Microbiology 8: 802-813 (2010).
Pulliam J.R.C. &. Dushoff . Ability to replicate in the cytoplasm predicts zoonotic transmission of livestock viruses. J Infect Dis 199(4): 565-568 (2009).
Regoes R.R. et al. Evolution of virulence in a heterogeneous host population. Evolution 54(1): 64-71 (2000).
Rigaud T. et al.Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc R Soc B 277(1701): 3693-3702 (2010).
Romanova L.I. Microevolution of tick-borne encephalitis virus in course of host alternation. Virology 362(1): 75-84 (2007).
Schmidt K.A. & R.S. Ostfeld. Biodiversity and the dilution effect in disease ecology. Ecology 82(3): 609-619 (2001).
Smith K.F. et al. Evidence for the role of infectious disease in species extinction and endangerment. Conserv Biol 20(5): 1349-1357 (2006).
Swaddle J.P. &S.E. Calos. Increased avian diversity is associated with lower incidence of human West Nile virus infection: observation of the dilution effect. PLoS ONE 3(6): e2488 (2008).
Swinton J. et al. Persistence thresholds for phocine distemper virus infection in harbour seal Phoca vitulina metapopulations. Journal of Animal Ecology 67: 54-68 (1998).
Taylor L.H. et al. Risk factors for human disease emergence. Phil Trans R Soc Lond B 356(1411): 983-989 (2001).
van Riper III C. et al.. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecological Monographs 56(4): 327-344 (1986).
Vasilakis N. Mosquitoes put the brake on arbovirus evolution: experimental evolution reveals slower mutation accumulation in mosquito than vertebrate cells. PLoS Pathog 5(6): e1000467 (2009).
Woolhouse M.E.J. et al. Population biology of multihost pathogens. Science 292(5519): 1109-1112 (2001).





























