« Prev Next »

How species have populated our planet has long fascinated biologists, particularly since the functional mechanism of evolution was described by Darwin (1859). In most regions of the Earth, one can observe a variety of similar organisms with different ecologies or morphologies. For example, in North America, you could easily find one of the 35 species of ratsnakes from the tribe Lampropletini (Figure 1) that vary noticeably with respect to shape, color, and body length. Species in this group exhibit a wide range of ecologies, which consist of diets of reptile eggs, other snakes, lizards, birds, and small mammals, as well as habitat preferences, which include deserts, grasslands, tropical rainforests, and northern deciduous forests. With such extreme diversity of form and ecology, one might ask, how did all of this variation arise over time? Do these species originate and accumulate morphological and ecological differences at a steady rate, or, given vacant niches, do they fill them rapidly?

Paleontologists first indicated that a large number of morphologically diverse species from a related group usually appear rapidly in the fossil record (Simpson 1944, Sloss 1950, Foote 1993). This pattern is often followed by a leveling off of the number of new species from the group present in subsequently younger stratigraphic layers, ultimately leading to an eventual decline in taxa through time as extinction rises. Describing the mechanism associated with this pattern, Simpson (1953) suggested that taxa from a related group will fill vacant niches by speciating rapidly, producing many diverse forms with very different ecologies. When niches are exhausted, then the rate of speciation will begin to slow. This pattern of early, rapid speciation is thought to be caused by ecological opportunity (EO) — a relaxation of natural selection in the face of abundant evolutionarily available resources (Schluter 2000, Glor 2010, Yoder et al. 2010). Because of this, many of Earth's most extraordinary examples of adaptive radiations include taxa that exploit resources which, under many conditions, would be utilized only by unrelated taxa. Examples of this include radiations of finches on remote islands into niches usually filled by warblers and woodpeckers on the mainland, and the marsupial mammals of Australia that have diversified into homologous ecological roles occupied by the placental mammals found elsewhere in the world (Schluter 2000, Phillips et al. 2006).
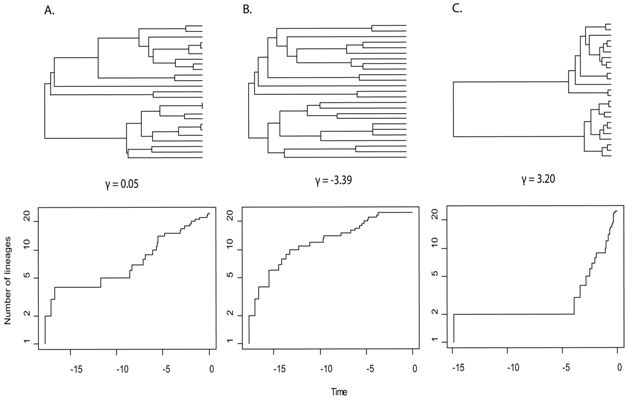
While the pattern was first noticed in the fossil record, evolutionary biologists have also uncovered the effects of ecological opportunity in extant species. Using time calibrated molecular phylogenies, researchers have examined the pace of speciation through time (Figure 2). If EO was available at the origin of a group, then we expect the density of most nodes (speciation events) to be clustered early in the group's history (Purvis et al. 2009). A statistic that examines this, γ, runs the spectrum from negative (representing an early burst of speciation) to 0 (even speciation through time) and positive (late bursts of speciation or, alternatively massive, early extinction; Pybus & Harvey 2001). Many examples using molecular phylogenies in a variety of organisms, including birds, lizards, and snakes (Harmon et al. 2008, Phillimore & Price 2008, Burbrink & Pyron 2010) show the signature of early bursts of speciation associated with ecological opportunity. While interpretations of data and mathematics associated with analyzing these phylogenetic patterns are improving rapidly, there is concern that extinction and poor species sampling may yield incorrect patterns for estimates of diversification (speciation minus extinction) through time (Purvis et al. 2009, Rabosky 2010). However, the overwhelming signal of early and rapid diversification is still common in cases where EO is suspected (Phillimore & Price 2009).
It seems clear that molecular phylogenies and the fossil record can point to the signature of early rapid speciation. But do morphological differences also change early and rapidly in a group's history? Paleontological research has shown that the morphologies of trilobites and echinoderms change most rapidly early in the history of each group (Foote 1993). Recently, phylogenetic comparative methods have been developed to examine the effects of ecological opportunity on the diversification of morphology in extant lineages (Harmon et al. 2003). These tests rely on the idea that organisms will fill different ecologies rapidly and will occupy very different islands of morphological diversity. Within these islands, morphological diversity is expected to be low. Results from studies employing these methods indicate that taxa with early bursts of speciation will partition morphological disparity among rather than within subclades (Harmon et al. 2003, Burbrink & Pyron 2010). This suggests that taxa that diversify early in their histories will fill available niche space and leave little opportunity for subsequent ecological diversification within subclades (Figure 3). Yet some comparative research contradicts the expected pattern of bursts of morphological diversification early in a taxon's history, instead suggesting that morphological evolution within an adaptive radiation is constrained by selection (Harmon et al. 2010).

Ecological opportunity and rapid speciation relies on vacant niches, but we have yet to discuss how these open ecologies arise. There are four circumstances where open niches would be available for an organism, all of which were first proposed by Simpson (1953): 1) origin of newly available resources, 2) invasion of a novel landmass, 3) extinction of predators or competitors, and 4) evolution of a key innovation. Additionally, these sources could act together to produce an adaptive radiation. In the first source of EO, the diversification of one group of organisms can in turn create ecological opportunity for another unrelated group of species — in that the process of the former's radiation may yield an untapped niche for the latter (Losos 2010). This is illustrated in recent work on the coevolution of weevils and the flowering plants (McKenna et al. 2009). Weevils are a diverse group of herbivorous beetles (~62,000 species) with extremely divergent ecologies. The authors sought to clarify higher-level relationships and divergence times within weevils to examine the evolutionary history of weevil-angiosperm associations. A time-calibrated phylogeny was created using fossil and molecular sequence data. Divergence times of major weevil clades were plotted against previous angiosperm dominance over the time period of the Cretaceous. Their results suggest that a major increase in angiosperm and associated weevil diversity arose approximately simultaneously throughout the mid-Cretaceous (McKenna et al. 2009).
The invasion of a novel habitat depauperate in competing taxa and predators provides yet another, and perhaps one of the most common sources, of EO. Colonization of remote islands and subsequent diversification on these vacant landmasses, such as in Galapagos finches and the anoles of the Greater Antilles, are classic examples of EO via invasion of novel habitats. Other sources include habitats created by the uplift of mountain ranges (Hughes & Eastwood 2006) or dispersal to an entire depauperate continental landmass (Burbrink & Pyron 2010). The study of EO at continental scales can be a daunting task, yet it may be the source of much of the diversity of life, as these landmasses provide the largest area and usually the most diverse habitats. Returning to our example in the beginning of this article, the diverse New World ratsnakes have recently been used to address hypotheses of ecological opportunity. This group diversified, both in terms of species numbers and morphological specialization, early in their history, following their colonization of North America from an Asian ancestor approximately 24 million years ago (ma). This colonization occurred when the New World snake fauna was largely underrepresented by similarly advanced snakes (Burbrink & Pyron 2010). These elevated, early diversification rates slowed drastically through time. The authors demonstrated that the saturation of ecological space may have lead to density dependent effects on diversification as ecological opportunity declined (Burbrink & Pyron 2010). The results of this work indicate that invasion of a novel land mass may foster rapid diversification, and that later competition, as more species arise, may influence the evolution of morphology (Burbrink & Pyron 2010) further supporting hypotheses of EO.
It has long been noted that clades will often rapidly diversify following an event where competitors or predators are driven to extinction-with extreme cases resulting in complete reorganization of ecological communities (Wolfe & Upchurch 1986, Smith et al. 2010). A good example of this third source of EO is the Cretaceous/Tertiary (K/T) global mass extinction event that effectively ended 76% of life on Earth and the subsequent rise of mammals, although some authors suggest that the diversification of mammals occurred much later (Bininda-Emonds 2007). This event provided the ecological opportunity for mammals to diversify into many ecological roles and increase in body size (Smith et al. 2010). Recently researchers analyzed data from the fossil record to examine the maximum size for land mammals across continents, lineages, and ecological guilds through time. Results show that maximum size leveled off after 40 million years of diversification and remained constant thereafter. The authors indicated that a multitude of niches became available and were filled by the diversification of mammals following the extinction of non-avian dinosaurs. Furthermore, the authors noted that the radiation of mammals resulted in convergence in both maximum body size and in ecologies, because similar niches were filled by different phylogenetic lineages at different times and in different areas (Smith et al. 2010).
The evolution of a key innovation has also been proposed as a mechanism for organisms to exploit new resources, thus gaining access to ecological opportunity (Schluter 2000). A key innovation can be defined as a newly evolved trait that allows a taxon to interact with the environment in a novel way without a specific change in the external environment (Losos 2010, Yoder et al. 2010). A number of traits have been proposed as key innovations leading to adaptive evolution, including the origins of wings in birds, bats, and pterosaurs, the adhesive toe pads of geckos, herbivory in insects, the nectar spurs of many angiosperm groups, and parity mode in squamates (Hodges & Arnold 1995, Schluter 2001, Lynch 2009, Losos 2010). The hypothesis that viviparity was a key innovation to exploit temperate habitats featuring drastically contrasting seasons was tested using phylogenetic methods to infer diversification rates across clades of viviparous and oviparous groups of vipers (Lynch 2009). It was found that viviparous clades diversified at a constant rate through time, whereas the diversification of oviparous groups declined at the onset of the cooler, Oligocene epoch. This global decrease in temperature was directly responsible for the decreased rate of diversification of oviparous clades of vipers (Lynch 2009). The results suggest that viviparity offered a buffer for live-bearing species against the potentially negative effects of global cooling and therefore was a key innovation that promoted the diversification of live-bearing vipers in cooler climates (Lynch 2009).
Adaptive radiation through the process of ecological opportunity accounts for much of the diversity of life, including both extinct and extant taxa. The idea of ecological opportunity acting as the trigger of adaptive radiation has been demonstrated in numerous examples using both living and extinct organisms. Ecological opportunity permits a group to experience rapid diversification in species number and morphological attributes. Additionally, morphological disparity in subclades is expected to be reduced early in the history of a group as ecological space is partitioned among these subclades. Unfortunately, tests of diversification have been carried out in a rather limited number of taxonomic groups. Future research will likely sample more taxa as well as more genes (i.e., through next-generation sequencing) in order to better reconstruct robust hypotheses of a taxon's evolutionary history. Although robust molecular phylogenies are essential to determining changes in diversification rates, they may be blind with respect to diversity trajectories and rates of extinction (Quental & Marshall 2010). Therefore studying the time course of diversification requires greater integration of molecular phylogenies with fossil data to gain a better understanding of the tempo and mode of diversification.
References and Recommended Reading
Bininda-Emonds, O. R. P. et al. The delayed rise of present-day mammals. Nature 446, 507-512 (2007).
Burbrink, F. T. & Pyron, R. A. How does ecological opportunity influence rates of speciation, extinction, and morphological diversification in new world ratsnakes (tribe Lampropeltini)? Evolution 64, 934-943 (2010).
Darwin, C. On the Origin of Species by Means of Natural Selection, or The Preservation of Favored Races in the Struggle for Life. London, UK: John Murray, 1859.
Foote, M. Discordance and concordance between morphological and taxonomic diversity. Paleobiology 19, 185-204 (1993).
Glor, R. E. Phylogenetic insights on adaptive radiation. Annual Review of Ecology, Evolution, and Systematics 41, 251-270 (2010).
Harmon, L. J. et al. Tempo and mode of evolutionary radiation in iguanian lizards. Science 301, 961-964 (2003).
Harmon, L. J. et al. The role of geography and ecological opportunity in the diversification of day geckos (Phelsuma). Systematic Biology 57, 562-573 (2008).
Harmon, L. J. et al. Early burst of body size and shape evolution are rare in comparative data. Evolution 64, 2385-2396 (2010).
Hodges, S. A. & Arnold, M. L. Spurring plant diversification: Are floral nectar spurs a key innovation? Proceedings of the Royal Society B: Biological Sciences 262, 343-348 (1995).
Hughes, C. & Eastwood, R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America 103, 10334-10339 (2006).
Losos, J. B. Adaptive radiation, ecological opportunity, and evolutionary determinism. The American Naturalist 175, 623-639 (2010).
Lynch, V. J. Live-birth in vipers (Viperidae) is a key innovation and adaptation to global cooling during the cenozoic. Evolution 63, 2457-2465 (2009).
McKenna, D. D. et al. Temporal lags and overlap in the diversification of weevils and flowering plants. Proceedings of the National Academy of Sciences of the United States of America 17, 7083-7088 (2009).
Phillips, M. J. et al. Combined mitochondrial and nuclear DNA sequences resolve the interelations of the major Australasian marsupial radiations. Systematic Biology 55, 122-137 (2006).
Phillimore, A. B. & Price, T. D. Density-dependent cladogenesis in birds. PLoS Biology 6, e71 (2008).
Pybus, O. G. & Harvey, P. H. Testing macro-evolutionary models using incomplete molecular phylogenies. Proceedings of the Royal Society B: Biological Sciences 267, 2267-2272 (2001).
Purvis, A. et al. "Temporal patterns in diversification rates," Speciation and Patterns of Diversity, 278-300. eds. R. K. Butlin, J. R. Bridle & D. Schluter Cambridge, UK: Cambridge University Press, (2009).
Quental, T. B. & Marshall, C. R. Diversity dynamics: Molecular phylogenies need the fossil record. Trends in Ecology & Evolution 25, 434-441 (2010).
Rabosky, D. L. Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816-1824 (2010).
Schluter, D. The Ecology of Adaptive Radiation. Oxford, UK: Oxford University Press, 2001.
Simpson, G. G. Tempo and Mode in Evolution. New York, NY: Columbia University Press, 1944.
———. The Major Features Of Evolution. New York, NY: Columbia University Press, 1953.
Sloss, L. L. Rates of evolution. Journal of Paleontology 24, 131-139 (1950).
Smith, F. A. et al. The evolution of maximum body size of terrestrial mammals. Science 330, 1216-1219 (2010).
Yoder, J. B. et al. Ecological opportunity and the origin of adaptive radiations. Journal of Evolutionary Biology 23, 1581-1596 (2010).
Wolfe, J. A. & Upchurch, G. R. Jr. Vegetation, climatic and floral changes at the Cretaceous-Tertiary boundary. Nature 324, 142-152 (1986).





























