« Prev Next »
The ecological interactions of parasites (defined here to include both macroparasites and microparasites) are often challenging to observe. Many live their lives secretively, in intimate contact with their host, but invisible to the outside world. With some notable exceptions (e.g., tapeworms), parasites also tend to be very small. It may be easy to assume then, that since parasites are generally inconspicuous, they play less important roles in community ecology than free-living organisms. Yet advances in the field of disease ecology have revealed that parasites are not only ecologically important, but can sometimes exert influences that equal or surpass those of free-living species in shaping community structure. In fact parasitism is more common than traditional predation as a consumer lifestyle (De Meeûs & Renaud 2002), and arguably represents the most widespread life-history strategy in nature (Price 1980). Parasites also influence host behavior and fitness, and can regulate host population sizes, sometimes with profound effects on trophic interactions, food webs, competition, biodiversity and keystone species. These interactions suggest that parasites are integral components in shaping community- and ecosystem structure.
Parasitism and Trophic Interactions
Parasites can function as both predators and prey. Parasites that feed on hosts engage in a special type of predation (Raffel et al. 2008). Alternatively, parasites can also serve as important sources of prey (Figure 1). For example, predators on islands in the Gulf of California, including lizards, scorpions and spiders, are one- to two orders of magnitude more abundant on islands with sea bird colonies because they feed on bird ectoparasites (Polis & Hurd 1996). Predators also inadvertently consume parasites during the consumption of infected hosts (Johnson et al. 2010). When macroparasites are relatively large, such as nematodes in the gut of vertebrate hosts, the contributions of parasites to the diet of predators can be significant. The roles of parasites as predators and prey suggest that considerable amounts of energy may directly flow through parasites in food webs, despite their small size and cryptic nature.
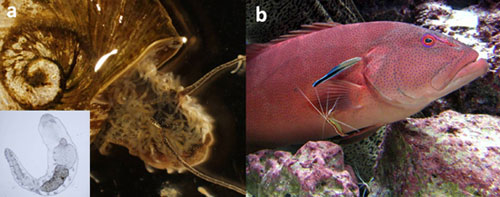
In some cases, predation can serve as a vehicle of transmission, allowing a parasite with a complex life cycle to move from one host to another. Parasites that infect new hosts via trophic transmission frequently alter their host's behavior or morphology in ways that increase predation risk, thereby aiding the parasite in reaching the next host in its life cycle (Poulin et al. 2005). For example, estuarine killifish infected with the trematode Euhaplorchis californiensis exhibit erratic swimming behavior that ultimately makes them up to 30 times more susceptible to bird definitive hosts (Lafferty & Morris 1996). Another trematode endoparasite, Ribeiroia ondatrae, causes amphibians to develop severe limb deformities, including extra or missing limbs (Johnson et al. 1999), which impair the host's ability to jump and swim, and presumably make them more susceptible to predation by bird definitive hosts (Figure 2). The roles of parasites in predator-prey interactions are rarely obvious, yet they may influence the outcome of trophic interactions at the community scale.

Parasitism, Food Webs, and Ecosystem Energetics
For decades, parasites were omitted from the study of food web ecology based on the assumption that they contributed negligible biomass to ecosystems. Measuring biomass, or productivity, allows us to quantify contributions of organisms to ecosystem energetics. However, when parasite biomass was actually measured on an ecosystem scale, the results challenged the notion that parasites are unimportant in ecosystem energy flow. In some estuarine systems, the biomass of parasites is comparable to that of top predators (Kuris et al. 2008). Yearly productivity of trematode parasites, for example, was higher than the biomass of birds. Similarly, the estimated biomass of plant fungal pathogens was comparable to that of herbivores in experimental grassland plots in Minnesota (Figure 3; Mitchell 2003). In fact, top-down control by fungal pathogens was more important than herbivory in predicting grass biomass. These studies suggest that parasites can contribute significantly to ecosystem energetics and exert strong control over the biomass of primary producers. While much remains to be learned about the roles of parasites in food webs, the classical approach of omitting parasites from considerations of food web ecology could lead to serious errors in our understanding.

Parasitism, Competition, and Biodiversity
Parasites can influence biodiversity when they alter the outcome of competitive interactions between host species, a phenomenon termed parasite-mediated competition (Price et al. 1986). In some cases, this occurs when a tolerant host species amplifies a parasite's abundance, causing an indirect negative effect on a second, less tolerant host species. For example, the displacement of red squirrels by grey squirrels in Britain may have been facilitated by a parapoxvirus (Tompkins et al. 2003). The virus infects both species, but native red squirrels are highly susceptible, whereas invasive grey squirrels experience relatively minor negative effects. In this case, a microparasite has probably facilitated a biological invasion, thereby reducing local biodiversity by eliminating populations of one host species.
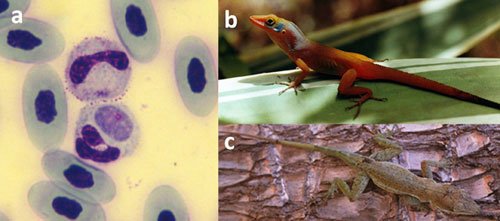
Parasites can also positively contribute to biodiversity by allowing a competitively inferior species to coexist with a dominant species. For example, Anolis gingivinus outcompetes Anolis wattsi everywhere on the Caribbean island of St. Maarten, except the isolated interior of the island. Both lizards host a malarial parasite, Plasmodium azurophilum, but the two lizards co-occur only where A. gingivinus is heavily parasitized (Figure 4). This suggests that malaria reduces the competitive ability of the dominant lizard, thereby allowing the competitively inferior lizard to coexist (Schall 1992). A similar outcome in a very different system occurs with the pathogenic soil oomycete Pythium and its plant hosts. The presence of a particular plant can change the composition of the local soil community such that the growth of that species is diminished, and other colonizing species are given a competitive advantage, which ultimately increases overall plant biodiversity (Mills & Bever 1998).
Parasites also influence biodiversity through the direct regulation of host populations. Even though parasites can cause disease, they rarely cause extinctions, because pathogen transmission is usually reduced at low host densities. However, important exceptions can occur, particularly in cases when pathogens invade naïve host populations, or when reservoir hosts allow parasites to persist despite low host densities. The emergence of the amphibian fungal pathogen, Batrachochytrium dendrobatidis (Bd), for example, represents a case of a parasite causing mass population declines, and even extinctions of frogs, on a global scale (Kilpatrick et al. 2010). Collectively, the examples described here illustrate how parasites may have opposing net effects on biodiversity, which depend on the context of the parasite-host relationship (e.g., whether host populations are naïve, and whether parasite transmission is density-dependent), and on whether parasites most negatively affect competitively dominant or competitively inferior species in a community.
Parasitism, Keystone Species, and Ecosystem Structure
Another example involves the introduction and subsequent removal of a viral disease called rinderpest in African ungulates. The virus was introduced to native ungulates from domestic livestock in 1890, and spread throughout the African continent within 10 years, reducing populations of buffalo and wildebeest (also known as gnus) by 80% in some regions. Vaccination of domestic cattle began in the 1950s (Plowright & Taylor 1967) and virtually eliminated the disease in wild African ungulates by 1968. The release of herbivores from parasite control had dramatic cascading effects on the ecosystem: populations of top carnivores, including lions and hyenas increased, and the productivity of grasses decreased (Sinclair 1979; Thomas et al. 2005). These examples of parasite introductions and removals provide a rare glimpse into how ecosystem structure can be dramatically altered when parasites regulate populations of functionally important host species.
Concluding Remarks
References and Recommended Reading
De Meeûs, T. & Renaud, F. Parasites within the new phylogeny of eukaryotes. Trends in Parasitology 18, 247-251 (2002).
Edmunds, P. J. & Carpenter, R. C. Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proceedings of the National Academy of Science, USA, 98, 4822-4824 (2001).
Elton, C. S. Animal Ecology. London, UK: Sidgwick and Jackson, 1927.
Johnson, P. T. J., Dobson, A. et al. When parasites become prey: Ecological and epidemiological significance of eating parasites. Trends in Ecology and Evolution 25, 362-371 (2010).
Johnson, P. T. J., Lunde, K. B. et al. The effect of trematode infection on amphibian limb development and survival. Science 284, 802-804 (1999).
Killpatrick, A. M., Briggs, C. J. et al. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends in Ecology and Evolution 252, 109-118 (2010).
Kuris, A. M., Hechinger, R. G. et al. Ecosystem energetic implications of parasite and free-living biomass in three esturaries. Nature 454, 515-518 (2008).
Laferty, K. D., Allesina, S. et al. Parasites in food webs: The ultimate missing links. Ecology Letters 11, 533-546 (2008).
Lafferty, K. D. & Morris, A. K. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77, 1390-1397 (1996).
Lafferty, K. D., Dobson, A. P. et al. Parasites dominate food web links. Proceedings of the National Academy of Science, USA, 103, 11211-11216 (2006).
Lessios, H. A. Mass mortality of Diadema antillarum in the Caribbean: What have we learned? Annual Review of Ecology and Systematics 19, 371-393 (1988).
Mills, K. E. & Bever, J. D. Maintenance of diversity within plant communities: Soil pathogens as agents of negative feedback. Ecology 79, 1595-1601 (1998).
Mitchell, C. E. Trophic control of grassland production and biomass by pathogens. Ecology Letters 6, 147-155 (2003).
Plowright, W. & Taylor, W. P. Long term studies of immunity in East African cattle following inoculation with rinderpest culture vaccine. Researches in Veterinary Science 8, 118-128 (1967).
Polis, G.A. & Hurd, S. D. Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. American Naturalist 147, 396-423 (1996).
Poulin, R., Fredensborg B. L. et al. The true cost of host manipulation by parasites. Behavioural Processes 68, 241-244 (2005).
Price, P. W. Evolutionary Biology of Parasites. Princeton, NJ: Princeton University Press, 1980.
Price, P. W., Westoby, M. et al. Parasite mediation in ecological interactions. Annual review of ecology and systematic 17, 487-505 (1986).
Raffel, T. R., Martin, L. B. et al. Parasites as predators: Unifying natural enemy ecology. Trends in Ecology and Evolution 23, 610-618 (2008).
Schall, J. J. Parasite-mediated competition in Anolis lizards. Oecologia 92, 58-64 (1992).
Sinclair, A. R. E. The eruption of the ruminants. In Serengetti: Dynamics of an Ecosystem. eds. Sinclair, A. R. E. & Norton-Griffiths M. (Chicago: University of Chicago Press, 1979): 82-103.
Sukhdeo, M. V. K. & Hernandez, A. D. Food web patterns and the parasite's perspective. In Parasitism and Ecosystems. eds. Thomas, F, Renaud, F. et al. (New York: Oxford University Press, 2005): 54-67.
Thomas, F., Bonsall, M. B. et al. Parasitism, biodiversity and conservation. In Parasitism and Ecosystems. eds. Thomas, F., Renaud, F. et al. (New York: Oxford University Press, 2005): 124-139.
Tompkins, D. M., White, A. R. et al. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecology Letters 6, 189-196 (2003).






























