« Prev Next »

Biogeochemical cycles describe pathways by which chemical elements move through both biotic (the biosphere) and abiotic compartments (the atmosphere, hydrosphere, and lithosphere) on Earth. Along with energy flows, biogeochemical cycles establish the relations among ecosystem compartments at local, regional and global scales. In these systems of inputs, outputs, sources and sinks, elements are moved from one part of an ecosystem (e.g., ocean, soil, atmosphere) where the element may temporarily accumulate to another, back and forth among organisms, and from living organisms to the abiotic environment and back again. In other words, chemical elements are cycled and reused within and among Earth's various compartments over and over again.
The biogeochemical cycles proceed through biological, geological and chemical interactions along hydrological, gaseous, and mineral "trade routes." Among the most ecologically important and well known are the element cycles of carbon (C), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S), as well as the water (H2O) cycle. One biogeochemical cycle that is often overlooked, however, is Earth's iron (Fe) cycle (Figure 1).
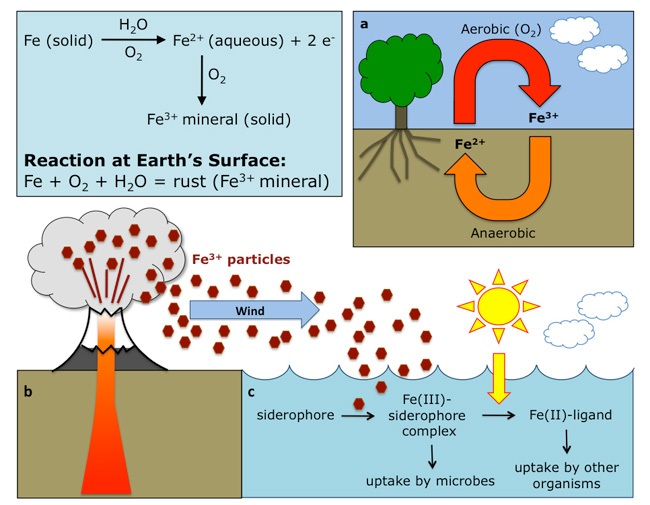
Iron composes more than 30% of the Earth's mass, and is a ubiquitous element found in the atmosphere, biosphere, lithosphere, and hydrosphere. It is one of the most abundant elements on Earth and among the most important elements in the biosphere (Morgan & Anders 1980). It is an essential element for countless cellular processes and metabolic pathways in both eukaryotic and prokaryotic organisms. Yet despite its abundance, iron can be in short supply for growing organisms because it changes its chemical form, in ways that govern its availability. In its pure state it is a reactive metal that oxidizes readily in the presence of oxygen. On Earth, iron exists in one of two oxidation states: reduced ferrous iron, depicted as Fe(II) or Fe2+, or oxidized ferric iron, depicted as Fe(III) or Fe3+). These states can be found in nature as solids in the form of Fe(III)- and Fe(II)-bearing minerals (Figure 2) or as ions (Fe3+ and Fe2+) dissolved in water. The amount of Fe in a body of water, and the prevalence of each oxidation state, are controlled by oxygen concentration, pH, and the biological activities of microorganisms and higher organisms. The biogeochemistry of exchange between iron's two forms has been amusingly referred to as Earth's "ferrous wheel."

On Earth when water comes into contact with solid iron in the presence of atmospheric oxygen, Fe is rapidly oxidized to Fe2+ via the reaction:
Fe (solid) → Fe2+ (aqueous) + 2e-
Hydroxide ions in the water then react with the Fe2+ ions to produce solid Fe(III)-minerals through the reaction:
Fe2+ (aqueous) + 2OH- (aqueous) → Fe(OH)2 (solid mineral made of ferric iron)
These Fe(III)-minerals are often powdery and red in color when dried, and are commonly referred to as rust. Different Fe(III)-minerals (Table 1 & Figure 2) will form under different environmental conditions (e.g., oxygen exposure, pH, presence of other ions). Oxidized, particulate forms of iron are poor sources for the growth of aquatic life because iron, in its solid form, is not readily available for organisms to use for growth and reproduction.
| Table 1. Common iron-containing minerals found on Earth | |||||
| Oxides | Oxy-hyrdoxides | Hydroxides | |||
| Hematite | α- Fe2O3 | Akaganéite | β-FeOOH | Bernalite | Fe(OH)3 |
| Maghemite | β-Fe2O3 | Feroxyhite | δ'-FeOOH | Fe(OH)2 | |
| γ-Fe2O3 | Ferrihydrite | Fe5HO8 + 4H2O | Green rust1 | ||
| ε-Fe2O3 | Goethite | α-FeOOH | |||
| Magnetite | Fe3O4 | Lepidocrocite | γ-FeOOH | ||
| Wüstite | FeO | Schwertmannite | δ-FeOOH | ||
| 1. In general, green rusts have the formula Fe3+x Fe2+y (OH)3x+2y-z(A-); where A- = Cl- or ½ SO42- | |||||
Numerous iron-containing minerals exist on Earth (Table 1 & Figure 2). These minerals are classified as Fe(III)-oxides (e.g., hematite), Fe(III)-oxyhydroxides and -hydroxides (e.g., goethite), Fe(II)-minerals (e.g., pyrite), or mixed Fe(II)-Fe(III) minerals (e.g., magnetite). The formation of these inorganic compounds initially involves the aerobic weathering of primary rocks in terrestrial and marine systems (Cornell & Schwertmann 2003). This process might be followed by redistribution between compartments (e.g., hydrosphere and lithosphere) and may involve mechanical transport by wind and/or water (Cornell & Schwertmann 2003). Iron-metabolizing microorganisms, which catalyze the oxidation or reduction of iron, also play a significant role in the cycling of iron in the environment and between compartments.
Fe(III)-minerals are characterized by low solubility at circumneutral pH, and thus exist as a solid in much of Earth's near-surface environments. However, in environments that are strongly alkaline or acidic, Fe(III)-minerals can dissolve because of the mineral's ability to act both as a base and as an acid. Fe(II)-minerals, on the other hand, are considerably more soluble at neutral pH, and thus readily dissolve to release Fe2+ ions to the surrounding environment. Fe(II)-minerals are only stable at neutral or alkaline pH in anoxic environments (i.e., when no oxygen is present). In an aqueous or moist environment, exposed to oxygen from Earth's atmosphere, Fe(II) rapidly oxidizes to Fe(III), with a half-life of several minutes (Stumm & Morgan 1996). The Fe(III) that is produced exists as an Fe3+ ion in acidic environments, or as solid Fe(III)-minerals (Figure 2) in neutral or slightly alkaline environments.
The movement of iron through the biosphere is controlled, at least in part, by the actions of plants. Although considered a micronutrient for plants, iron is an essential trace element required for the production of chlorophyll. Since iron uptake occurs at the tip of the roots, anything that interferes with this process will result in iron deficiency. An iron deficient plant will show a yellowish color in younger leaves. However, while small amounts are necessary for growth, iron can become toxic to plants. Iron toxicity is associated with large concentrations of Fe2+ in the soil solution (Becker & Asch 2005), and has been described as one of the major growth limiting factors for rice (Dobermann & Fairhurst 2000, Sahrawat 1979, Sahrawat 2004). Iron toxicity occurs in inundated soils with high iron-oxidizing activity, although the accumulation of organic acids, hydrogen sulfide and other reduction products might contribute to this toxicity (Sahrawat 2004).
Because iron is an important micronutrient used by most organisms, and is required for important cellular processes such as respiration, oxygen transport in the blood, photosynthesis, nitrogen fixation, and nitrate reduction, its bioavailability is of concern for Earth's living organisms, especially in aquatic ecosystems. This is because, despite its relatively high abundance on Earth, iron is a minor component of aquatic systems because of its relative insolubility in water at circumneutral pH. Research has demonstrated that most areas of the open ocean have surface trace metal concentrations in the nanomolar to picomolar range (Morel & Price 2003). That means one iron ion present for every fifty billion or trillion water molecules. At that vanishingly low concentration, iron is very often the limiting nutrient for primary production (e.g., photosynthesis) in large expanses of ocean (Behrenfeld & Kolber 1999, Coale et al. 1996, Hutchins & Bruland 1998, Martin et al. 1994).
In the upper ocean, dissolved iron frequently occurs in the form of complexes with strong ligands (Rue & Bruland 1995) presumably of biological origin (Hutchins et al. 1999, Kondo et al. 2008, Mawji et al. 2008). A ligand is an ion or molecule that binds to a metal, such as iron. A study by Barbeau et al. (2001), showed that Fe(III)-binding molecules synthesized by ocean-inhabiting microorganisms might facilitate the photochemical cycling of iron in ocean surface waters. Furthermore, their research demonstrated that key factors of the cycle included photolysis of the Fe3+-ligand complexes (e.g., siderophores, which are metal chelating molecules synthesized by some microorganisms), the reduction of Fe3+ to Fe2+, and oxidation of the ligand, thus increasing the bioavailability of iron for uptake by planktonic communities (Figure 1) (Barbeau et al. 2001). Therefore, the presence of microbial populations is key to an understanding of iron cycling in the ocean, because they produce the organic ligands that can strongly affect the solubility of iron, and thus the iron uptake mechanisms utilized by numerous marine organisms (Geider 1999).
Over the last ten years, the importance and effects of iron fertilization processes in the ocean has become a major field of interest, focusing primarily on dust deposition and marine processes. The importance of dust transport and deposition, and its connection between ocean biogeochemistry and global climate, is becoming more clear (Jickells et al. 2005). Dust (natural or anthropogenic in origin) and volcanic ash have been identified as two major sources of iron playing a role in the fertilization of the ocean (Figure 1). On one hand, desert dust aerosol is dominated by small particles (<10 μm) that allow long-range transport over thousands of kilometers (Ginoux et al. 2001). On the other hand, volcanic eruptions can expel ash several kilometers high into the atmosphere. Coarse particles fall in the vicinity of the volcano, while fine — often glassy — ash particles travel further distances where they can reach remote areas (Breitbarth et al. 2010). Although dust and volcanic ash have an important effect on oceanic ecology, the key to the process is how much iron is soluble (i.e., bioavailable) to ocean-dwelling organisms. It has been reported that the solubility of iron from anthropogenic dust is higher than other naturally occurring dusts (e.g., soil) (Journet et al. 2008). Similarly, recent studies have demonstrated that volcanic ash quickly releases bioavailable iron on contact with water (Duggen et al. 2007, Frogner et al. 2001). That is, the acid salts that are adsorbed to the volcanic glass, dissolve instantaneously, hence releasing both macronutrients and trace metals (e.g., iron) to become available for primary production in Earth's oceans (Frogner et al. 2001). As a result, large volcanic eruptions could lead to a significant increase in the primary production of organisms that inhabit Earth's oceans. This, in turn, could lead to a cooler planet as more CO2 (a greenhouse gas) is removed from Earth's atmosphere and sequestered in the biomass of these ocean-dwelling photosynthetic organisms. The result is a tantalizing negative relationship between iron in dust particles, and CO2 levels in different layers of Antarctic ice cores that span several ice ages. The connection was developed into the "Iron Hypothesis" by oceanographer John Martin, who summarized the importance of Fe to Earth's oceans and hence Earth's climate with the provocative claim, "Give me a half a tanker of iron and I'll give you the next ice age."
Acknowledgment
The support of US National Science Foundation (Grant Number EAR-0920299) is gratefully acknowledged.
References and Recommended Reading
Barbeau, K. et al. Photochemical cycling of iron in the surface ocean mediated by microbial Iron(III)-binding ligands. Nature 413, 409–413 (2001).
Becker, M. & Asch, F. Iron toxicity in rice-conditions and management concepts. Journal of Plant Nutrition & Soil Science 168, 558–-573 (2005).
Behrenfeld, M. J. & Kolber, Z. S. Widespread iron limitation of phytoplankton in the South Pacific Ocean. Science 283, 840–843 (1999).
Breitbarth, E. et al. Iron biogeochemistry across marine systems — Progress from the past decade. Biogeosciences 7, 1075–1097 (2010).
Coale, K. H. et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383, 495–501 (1996).
Cornell, R. M. & Schwertmann, U. "Introduction to the iron oxides." In The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. (New York Wiley-VCH, 2003): 1–7.
Dobermann, A. & Fairhurst, T. H. Rice: nutrient disorders and nutrient management. The International Rice Research Institute Manila, The Philippines, 2000).
Duggen, S. et al. Subduction zone volcanic ash can fertilize the surface ocean and stimulate phytoplankton growth: Evidence from biogeochemical experiments and satellite data. Geophysical Research Letters 34, L01612 (2007).
Duggen, S. et al. The role of airborne volcanic ash for the surface ocean biogeochemical iron-cycle: A review. Biogeosciences Discussions 6, 827–844 (2009).
Frogner, P., Gislason, S. R., & Oskarsson, N. Fertilizing potential of volcanic ash in ocean surface water. Geology 29, 487–490 (2001).
Geider, R. J. Biological oceanography — Complex lessons of iron uptake. Nature 400, 815–816 (1999).
Ginoux, P. et al. Sources and distributions of dust aerosols simulated with GOCART model. Journal of Geophysical Research 106, 20 (2001).
Hutchins, D. A. & Bruland, K. W. Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393, 561–564 (1998).
Hutchins, D. A. et al. Competition among marine phytoplankton for different chelated iron species. Nature 400, 858–861 (1999).
Jickells, T. D. et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308, 67–71 (2005).
Journet, E. et al. Mineralogy as a critical factor of dust iron solubility. Geophysical Research Letters 35, L07805 (2008).
Kondo, Y. et al. Organic Iron(III) complexing ligands during an iron enrichment experiment in the western subarctic North Pacific. Geophysical Research Letters 35, L12601 (2008).
Martin, J. H. et al. Testing the iron hypothesis in ecosystems of the Equatorial Pacific-Ocean. Nature 371, 123–129 (1994).
Mawji, E. et al. Hydroxamate siderophores: Occurrence and importance in the Atlantic Ocean. Environmental Science & Technology 42, 8675–8680 (2008).
Morel, F. M. M. & Price, N. M. The biogeochemical cycles of trace metals in the oceans. Science 300, 944–947 (2003).
Morgan, J. W. & Anders, E. Chemical composition of Earth, Venus, and Mercury. Proceedings of the National Academy of Sciences USA 77, 6973–6977 (1980).
Rue, E. L. & Bruland, K. W. Complexation of Iron(III) by natural organic-ligands in the Central North Pacific as determined by a new competitive ligand equilibration adsorptive cathodic stripping voltammetric method. Marine Chemistry 50, 117–138 (1995).
Sahrawat, K. L. Iron toxicity to rice in an acid sulfate soil as influenced by water regimes. Plant Soil 51, 143–144 (1979).
Sahrawat, K. L. Iron toxicity in wetland rice and the role of other nutrients. Journal of Plant Nutrition 27, 1471–1504 (2004).
Stumm, W. & Morgan, J. J. Aquatic Chemistry, Chemical Equilibria and Rates in Natural Waters, 3rd ed. New York, NY: John Wiley & Sons, 1996.





























