« Prev Next »

Organisms are inherently competitive, yet cooperation is widespread. Genes cooperate in genomes; cells cooperate in tissues; individuals cooperate in societies. Animal societies, in which collective action emerges from cooperation among individuals, represent extreme social complexity. Such societies are not only common in insects, mammals, and birds, but exist even in simple species like amoebas (Figure 1). Animal societies vary in structure from eusocial insect colonies with a single reproductive female supported by hundreds, thousands, or even millions of non-breeding workers, to cooperatively breeding groups of vertebrates with one or more breeders and a small number of non-breeding helpers. Given the diversity of social taxa, why do some species form complex societies, while other closely related species do not? Within these societies, why do some individuals attempt to reproduce, while others delay their own reproductive efforts to help raise the offspring of others? Determining the answers to these and other questions requires considering how and why groups form, and how individual behavioral roles are determined within groups.

The Costs and Benefits of Group Living
Group-living typically provides benefits to individual group members. For instance, most animals only have one pair of eyes to look for food or to watch for predators. Animals living in groups, however, benefit from many more pairs of eyes to provide vigilance or help forage. But living in groups may also confer costs to members. As individuals aggregate, they become more conspicuous to predators and competition for food can increase. Therefore, when deciding to join a group, individuals must weigh the cost-benefit ratio of living solitarily versus with others. When the benefits of living together outweigh the costs of living alone, animals will tend to form groups (Alexander 1974). Other benefits of group-living may include receiving assistance to deal with pathogens (i.e., grooming), easier mating opportunities, better conservation of heat, and reduced energetic costs of movements. Other costs of group-living may include increased attack rates by predators, increased parasite burdens, misdirected parental care, and greater reproductive competition.
Although many species form short-term, unstable groups (e.g., herds of wildebeest, colonies of gulls), some form long-term, stable social groups where interactions among members often appear to be altruistic. For example, when a ground squirrel sounds an alarm call to warn other group members of a nearby coyote, it draws the coyote’s attention and increases its own odds of being eaten (Sherman 1977). Similarly, when a meerkat forgoes reproduction and instead feeds the young of another group member, it reduces the number of offspring it will produce during its lifetime (Clutton-Brock et al. 2001). Scientists since Darwin have wondered why animals like these perform cooperative behaviors that may be detrimental to their own evolutionary fitness. One key to understanding such altruistic behavior came from Robert Trivers, who considered a hypothetical group of animals in which one individual is faced with the opportunity to take a small risk in order to provide a large benefit to another (Trivers 1971). Although choosing not to help is typically the best choice for an individual’s fitness in the short-term, it could mean that the individual will not receive reciprocal help from others when it is needed in the future. This provides incentive for altruistic behavior in situations where individuals interact repeatedly, which typically occurs when animals live in stable groups. For example, many primates like baboons live in large groups and form coalitions where such cooperative interactions are frequent.
Kin Selection and Living in Families
Altruistic behavior can benefit individuals even when it is not reciprocated. William Hamilton was among the first to realize this when he considered how altruistic behaviors might evolve in living organisms (Hamilton 1963). Based upon observations of social insects, he reasoned that the extent to which an individual is willing to help another should be determined by the degree to which those individuals are related. For example, Hamilton would predict that a person should be more willing to run into a burning building to rescue their own cousin than they should be to save an unrelated stranger. This is because relatives share many genes, and evolutionary fitness is determined by the fraction of an individual’s genes that enter the next generation’s gene pool, regardless of whether those genes come from that particular individual or from identical copies in its relative. If the cousin who is saved goes on to reproduce, then the altruistic act of saving that relative increases the evolutionary fitness of the rescuer by passing on shared genes.

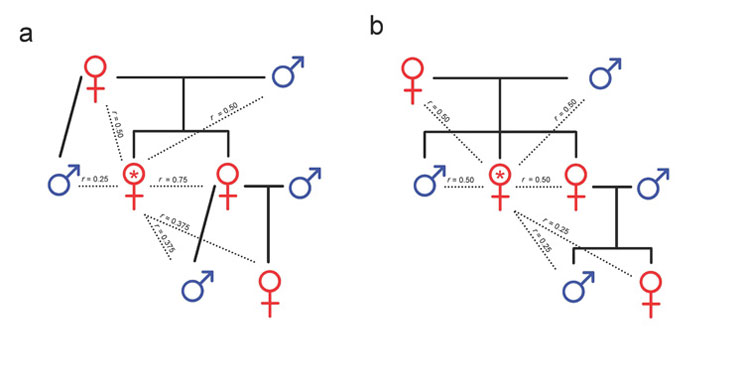
Kin selection theory is particularly relevant to social insect societies because many insects are haplodiploid. In haplodiploid species, an individual’s sex is determined not by the presence or absence of a sex chromosome, but instead by the number of copies of the genome in an individual’s cells. Diploid fertilized eggs containing two copies — one from each parent — become females, while unfertilized haploid eggs with only a single copy become males. Kin selection theory suggests that the sex-determined differences in relatedness among individuals (Figure 3) provide incentives for young females to stay at home and help raise their sisters. However, initial excitement over this explanation as a primary reason for sociality was tempered by observations of non-social haplodiploid species, as well as subsequent discovery of eusocial diploid species like termites.
Eusociality in Insect Societies
The enormous and highly complex societies formed by some insects have been especially important for studying the causes and consequences of sociality. These groups are called eusocial (i.e., truly social) because they share three key criteria: 1. cooperative care of young; 2. overlapping generations (i.e., parents and offspring cohabitating); and 3. a reproductive division of labor, often culminating in caste development (Wilson 1971). Eusociality was first formally described in ants by Wheeler (Wheeler 1928), and has since been shown to be widespread in numerous other insects, most notably in bees, wasps, and termites, as well as in some other taxa like snapping shrimp and even a rodent.
The coordination that makes a eusocial group successful depends upon the majority of group members forgoing personal reproduction. This allows workers to focus their efforts on specialized tasks, creating extraordinary efficiencies of scale. But why don’t workers reproduce? Are they being coerced into helping by queens, or are they helping voluntarily to gain the indirect fitness benefits of rearing relatives? Research suggests that helping behavior in insects may be a product of both coercion and voluntary actions. For example, evidence of coercion comes from honeybees, where individuals cannot choose their reproductive role and instead develop into either workers or queens based upon the nutrients they receive from others during their larval stage. However, there is also evidence from honeybees that workers voluntarily act to maximize their own fitness by rearing relatives. Although worker honeybees are unable to mate and produce diploid offspring, they are capable of laying unfertilized eggs that become males. Surprisingly, this rarely occurs when the queen is alive because workers police other workers, destroying any eggs laid. Such behavior maximizes the fitness of the policing worker by ensuring that her energy is devoted to raising the daughters of the queen (i.e., her sisters) and not her more distantly related nieces (Ratnieks & Visscher 1989).
Cooperative Breeding in Vertebrate Societies
Although few vertebrates are eusocial, many species live in complex, cooperatively breeding societies in which more than two individuals care for young. Alexander Skutch (Skutch 1935) made the first observations of cooperative breeding in birds, but subsequent biologists have reported the behavior in a variety of other taxa, including many mammals and fish. In addition to breeders, cooperative groups often contain a variable number of non-breeding auxiliaries, or helpers, that aid in raising the offspring of others. Unlike the workers of many social insect societies, vertebrate helpers are totipotent and retain the ability to reproduce throughout their lives. In most, but not all, cooperatively breeding species, helpers tend to be related to the breeders and therefore realize the indirect fitness benefits of raising relatives. In this way, the kin structure of vertebrate societies is similar to that of insect societies, even though group sizes are generally an order of magnitude smaller in vertebrates. Cooperative breeding is especially common in birds, including many well-studied species like Florida scrub jays and white-fronted bee-eaters, and mammals, including many rodents and carnivores.
Whereas kin selection likely underlies the evolution of many cooperatively breeding societies, it alone cannot explain why some species are social, while other closely related species are not. Biologists have long realized that the environment in which a population lives might influence whether or not its members live socially. To breed successfully, animals need a variety of resources including territories, food, and even mates. Without such resources, the likelihood of reproducing successfully is low, and so remaining with one’s family to help raise relatives may be a better option for maximizing fitness than trying to breed unsuccessfully elsewhere (Emlen 1982). In many species of birds, mammals, and fish, younger individuals often delay finding their own breeding territories for a few years until a better opportunity arises, instead remaining at home to gain valuable experience and parenting skills in addition to the indirect benefits of raising relatives. Similarly, when individuals live in hostile and unpredictable climates, cooperative breeding may be a conservative strategy to maximize fitness when breeding conditions cannot be accurately predicted from year to year (Rubenstein & Lovette 2007). Thus, cooperative breeding can be thought of as a ‘best of a bad job’ strategy to maximize fitness, either when opportunities for independent breeding are limited or when breeding conditions are uncertain.
Conclusion and Future Directions
Cooperation and sociality are widespread in animals. Seemingly altruistic behaviors, like raising the offspring of others instead of trying to reproduce, can largely be explained by the shared genetic heritage between interacting individuals. Most complex animal societies are actually families in which group members are related and therefore share a high proportion of their genes. The cooperative and often complex collective action that arises from such family groups is a product of the interaction of individuals seeking to maximize their own evolutionary fitness.
Genetic structure clearly influences the evolution of animal sociality. But do genes themselves influence an individual’s altruistic behavior? As new genomic tools become available, one of the challenges in the study of animal societies will be to determine how, and to what extent, individual genes underlie social behavior. For example, recent studies in wasps suggest that differences in the expression of a relatively small number of genes may be linked to large social differences in closely related species (Toth et al. 2007). Over the next decade, new research may determine if similar genetic mechanisms underlie social behavior in different types of species, including both invertebrates and vertebrates.
References and Recommended Reading
Alexander, R. The evolution of social behavior. Annual Review of Ecology and Systematic 5, 325-383 (1974).
Clutton-Brock, T. H, Brotherton, P. H. M. et al. Cooperation, control, and concession in meerkat groups. Science 291, 478-481 (2001).
Emlen, S. T. The evolution of helping 1: an ecological constraints model. The American Naturalist 119, 29-39 (1982).
Hamilton, W. D. The evolution of altruistic behavior. The American Naturalist 97, 354-356 (1963).
Maynard Smith, J. Group selection and kin selection. Nature 201, 1145-1147 (1964).
Ratnieks, F. L. W. & Visscher, P. K. Worker policing in the honeybee. Nature. 342, 796-797 (1989).
Rubenstein, D. R. & Lovette, I .J. Temporal environmental variability drives the evolution of cooperative breeding in birds. Current Biology 17, 1414-1419 (2007).
Sherman, P. Nepotism and the evolution of alarm calls. Science 197, 1246-1253 (1977).
Skutch, A. F. Helpers at the nest. Auk 52, 257-273 (1935).
Toth, A. L., Varala, K. et al. Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 318, 441-444 (2007).
Trivers, R. L. The evolution of reciprocal altruism. The Quarterly Review of Biology 46, 35-57 (1971).
Wheeler, W. M. The Social Insects: Their Origin and Evolution (Harcourt, Brace, New York, 1928).
Wilson, E. O. The Insect Societies. Cambridge, MA: The Belknap Press of Harvard University Press, 1971.






























