« Prev Next »

Populus tremuloides, the quaking aspen of the North American continent, stands as one of the most easily recognized, most beautiful and most admired of all tree species. In order to help appreciate this magnificent species, we here highlight some of its key biological attributes.
Perhaps most impressively, along with its very similar sister species of European and Asian distribution — the Eurasian aspen (Populus tremula) — these two species occupy the broadest range of any tree species in the world. Why is that so?
Quaking aspen can be found from Alaska to Mexico and from Vancouver to Maine (Jones et al. 1985; Mitton & Grant 1996). In the north-central part of the continent, quaking aspen occurs at almost any elevation, while in the southern part of the US and in Mexico, it tends to be found only at higher elevations. A similar distribution pattern exists for Populus tremula in Europe and Asia. Admirers frequently note the striking white bark of quaking aspen. This bark lives and carries out photosynthesis, attributes that make it unique among North American trees and likely contribute to its impressive geographic range (Figure 1).

Aspen drops its leaves in winter but, of course, remains alive and thus requires metabolic energy. The soft tan to greenish hues often visible in aspen bark mark an important photosynthetic capability provided by the differing levels of chloroplasts. Stem photosynthesis contributes significantly to aspen’s over-wintering survival capabilities (Bervieller et al. 2007; Foote et al. 1978; Knowlton et al. 1976). The disadvantages of this type of bark include low fire resistance, ease with which people carve their initials in it and attractiveness as a food source for elk, numerous insects and fungi.
Aspen form individual patches comprised of numerous stems, termed ramets, each with its own trunk, branches, leaves and a shared root system (Figure 2). All of these structures arose from a single aspen seed, often in the very distant past; while these patches remain connected via root systems, they comprise a single clone. If the root system between patches is severed, the patches form physiologically separate entitites but are generally still considered part of the same clone given that they are composed of genetically identical patches and parts, having been produced vegetatively. The boundaries of different clones stand out most clearly in the early spring when flowering and leafing-out occur (in that order). Aspen occur as males and females separately (dioecious), unlike the majority of tree species, which support both male and female reproductive parts on each individual (monoecious or hermaphroditic).
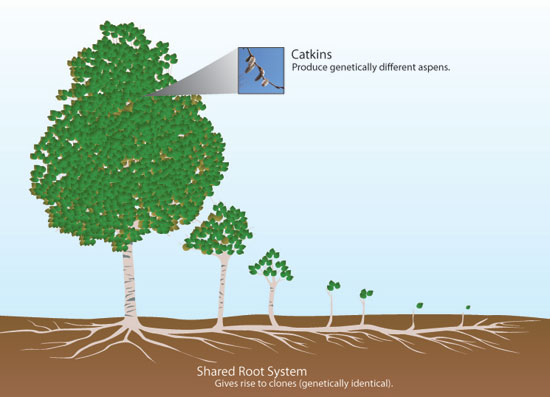
In early spring, an aspen clone will produce small, inconspicuous strings of reproductive parts called catkins, which are either male and produce pollen or female and produce eggs. Huge numbers of viable, tiny seeds mature and float off from the female on a tangle of cotton-like seed hairs that catch air currents, sometimes traveling great distances. Immediately after shedding their pollen and seeds, the clones then produce leaves that usually appear at the same time in all stems of a given clone. This time of the spring is when the boundaries of aspen clones stand out most visibly and reliably.
However, most aspen watchers tend to focus on the brilliant colors of aspen in the late fall prior to dropping their leaves (Figure 3). The dramatic visual show in the Rocky Mountains attracts many "leaf peepers" who find the brilliant gold, yellow, rose and even red leaf colors especially striking set against the dark green of their evergreen neighbors. But these color patches do not mark clonal boundaries as reliably as does leafing in the early spring because the chemical processes that produce the colors tend to be very sensitive to local micro-climate conditions such as aspect (whether north or south facing), soil moisture, etc. (personal observations). A single clone may exhibit multiple colors simultaneously. In aspen, all the pigments that give rise to these glorious colors can be found in the leaf from spring all the way through fall (see here). As summer begins to end and overnight temperatures drop, the aspen begin to first break down the green chlorophyll molecules that dominate the spring through summer color. The other pigment molecules — there all summer — then become more and more visible. This process reveals the golds, yellows and reds allowing aspen to really show their stuff until their leaves drop (Vogel 1993).

For many years, most western forest ecologists thought aspen reproduction from seeds was so rare as to warrant a publication of a single found seedling (Ellison 1943). However, it turns out that successful establishment of aspen via seeds occurs more frequently than previously thought (Mock et al. 2008). The ability of aspen to produce whole stands of "trees" vegetatively provides yet another key element in explaining the species’ ability to occupy huge geographic ranges.
There are several benefits of asexual expansion — spreading via roots which then send up shoots. As is true of other species of vegetatively-spreading plants (DeByle 1964; de Kroon & van Groenendael 1997; Jónsdóttir & Watson, 1997), one part of a clone might be near an important water source and thus "share" that water with parts of the clone (i.e., the other ramets in the clone) while those in a drier area may have greater access to a vital soil nutrient (e.g., phosphorous) that can also be distributed around the clone (Hansen & Dickson, 1979; Peltzer 2002; Pitelka & Ashmun 1985).
Quaking aspen also tends to be a disturbed habitat species, meaning it often lives where avalanches, mudslides and fires occur frequently. So both the regenerative capability and the clonal reproductive capability allow aspen to initially establish or to re-establish into an area after disturbances occur, especially spectacular ones like the 1988 fires in Yellowstone National Park (Romme et al. 1997). Careful observers of steep mountainous slopes along the Rocky Mountain Cordillera will regularly see avalanche and mudslide tracks populated by young, light-green aspen clones re-colonizing those spaces with shoot densities up to 30,000 per acre (Jones et al. 1985). Similar patterns often follow forest fires. Rarely will a fire burn hot enough to kill the entire root system from which these stems arise, so an individual clone may occupy a given space and be completely wiped out on the surface but re-grow from the root system many times.

Given its size, it may also be very old, perhaps 80,000 years, but good dating of the time the original, tiny seed germinated and established this clone lies beyond current scientific capabilities. Plausible estimates have been offered ranging from several thousands to a million years in age, although recent molecular work argues that these may be overestimates (Barnes 1975; Mock et al. 2008). Whatever its age, Pando certainly represents one of the most remarkable individuals among all living organisms.
In order to have this single genotype occupy this space for those huge spans of time, the external environment must have had just the right balance of disturbance and stability. If an aspen stand does not experience periodic disturbances such as fire or avalanche, more shade-tolerant conifers tend to establish and shade out the high-light-requiring aspen stems. If the disturbances are too frequent, then the clone could not establish and spread to this extent.
Clone structure varies with geography but also varies due to the strong influences of rainfall and relative humidity. The largest clones generally occur in semi-arid environments such as the mountains of the western and southwestern US. Clones tend to be smaller in areas where the climate supports seed germination and establishment somewhat more readily (e.g., the eastern parts of North America and the upper Midwest).
The last particular attribute of quaking aspen we here highlight as important in contributing to its ability to occupy huge ranges derives from the comparatively high level of genetic variability among clones (Cheliak & Dancik 1982; Kanaga et al. 2008; Madritch et al. 2009; Mock et al. 2008). These interclonal levels of variability provide the raw material for evolutionary change across generational times. The large number of seeds produced from genetically variable sources generates an enormous array of potentially successful genotypes for establishment in newly opened areas and probably takes place at higher rates than previously thought by forest ecologists. These insights derive from the application of modern molecular techniques to quaking aspen in the field.
With only a bit of whimsy, we have saved one of the most obvious attributes for last: Why do quaking aspen leaves quake and tremble? The leaves of this species quake, shake and tremble in the presence of even the slightest breeze due to the physical structure of the leaf stem (petiole) which traces a flat, oblong, elliptical pattern when viewed in cross section (i.e., perpendicular to the stem itself) so it has strength in one dimension (the long part of the ellipsis) and minimal strength in the second dimension (the narrow part of the ellipsis), so even a gentle wind causes shaking, quaking and trembling. We understand this phenomenon very well mechanically, yet a deeper question can be posed: Why does the petiole develop this way? Plant physiologists have pointed out several consequences of the trembling leaf behavior to include minimizing the risk of too much sunlight on the photosynthetic apparatus (photoinhibition), reducing the risk of overheating in intense, high elevation sunlight and improving photosynthetic rates by keeping a fresh supply of carbon dioxide near the leaf surface where the plant takes up that compound. Taking a different approach, one of our students did a small scale independent study several years ago where she identified matched pairs of aspen leaves and stabilized one with tubing to reduce its ability to tremble, then measured the amount of leaf damage due to insects near the end of the summer, comparing the leaves which could tremble with ones that could not. She found the insect damage to the ‘fixed’ leaves was, on average, about 27% higher in the stabilized members of the pairs.
This tree species seems to almost have it all: powerful, opportunistic, sexual reproduction, long-distance seed dispersal, effective vegetative spread, clonal reproduction, regeneration from roots, high levels of genetic variability, living bark and a potentially enormous life span.
We anticipate that this glorious, golden, and gigantic species will provide great pleasure for future admirers of its beauty as well as revealing a rich trove of scientific insights as we learn from its enormously successful colonization of a huge geographic range.
References and Recommended Reading
Barnes, B. V. The clonal growth habit of American aspens. Ecology 47, 439-447 (1966).
Barnes, B. V. Phenotypic variation of trembling aspen of western North America. Forest Science 21, 319-328 (1976).
Berveiller, D. & Damesin, D. Interspecific variability of stem photosynthesis among tree species. Tree Physiology 27, 53-61 (2007).
Cheliak, W. M., Dancik, B. P. Genic diversity of natural populations of a clone forming tree. Populus tremuloides. Canadian Journal of Genetics and Cytology 24, 611-616 (1982).
DeByle N. V. Detection of functional intraclonal aspen root connections by tracers and excavation. Forest Science 10, 386-396 (1964)
de Kroon H. & van Groenendael, J. The Ecology and Evolution of Clonal Plants. Leiden, The Netherlands: Backhuys Publishers, 1997.
DeWoody, J., Rowe, C. A. et al. "Pando" lives: molecular genetic evidence of a giant aspen clone in central Utah. Western North American Naturalist 68, 493-497 (2008).
Ellison, L. A natural seedling of western aspen. Journal of Forestry 41, 767-768 (1943).
Foote, K. C. & Schaedle, M. Diurnal and seasonal patterns of photosynthesis and respiration by stems of Populus tremuloides Michx. Plant Physiology 58, 651-655 (1976).
Foote, K. C. & Schaedle, M. The contribution of aspen Bark photosynthesis to the energy balance of the stem. Forest Science 24, 569-573 (1978).
Grant, Michael C. "The Trembling Giant." Discover Magazine (October 1993).
Grant, M. C., Mitton, J. B., et al. Even larger organisms. Nature 360, 216 (1992).
Hansen, E. A. & Dickson, R. E. Water and mineral nutrient transfer between root systems of juvenile Populus. Forest Science 25: 247-252 (1979).
Jónsdóttir, I. S. & Watson, M. A. Extensive physiological integration: an adaptive trait in resource-poor environments?. In The Ecology and Evolution of Clonal Plants, eds. de Kroon, H. & van Groenendael, J. (Leiden: Backhuys Publishers, 1997) 109-136.
Kanaga, M. K., Ryel, R. J. et al. Quantitative-genetic variation in morphological and physiological traits within a quaking aspen (Populus tremuloides) population. Canadian J. of Forest Research 38, 1690-1694 (2008).
Madritch, M. D., Greene, S. L. et al. Genetic mosaics of ecosystem functioning across aspen-dominated landscapes. Oecologia 160, 119-127 (2009).
Mitton, J. B. & Grant, M. C. Genetic variation and the natural history of quaking aspen. BioScience 46, 25-31 (1996).
Mock, K. E., Rowe, C. A. et al. Clonal dynamics in western North American aspen (Populus tremuloides). Molecular Ecology 17, 4827-4844 (2008).
Peltzer, D. A. Does clonal integration improve competitive ability? A test using aspen (Populus tremuloides (Salicacae) invasion into a prarie. American Journal of Botany 89, 494-499 (2002).
Pitelka L. F. & Ashmun, J. Physiology and integration of ramets in clonal plants. In Population biology and evolution of clonal organisms, eds. Jackson, J. B. C., Buss, L. W., & Cook, R. E. (New Haven, Connecticut: Yale University Press, 1985): 399-437.
Romme, W. H., Turner, M. G. et al. A rare episode of sexual reproduction in aspen (Populus tremuloides) following the 1988 Yellowstone fires. Natural Areas Journal, 17, 17-25 (1997).
Vogel, Steven. When leaves save the tree. Natural History 102, 48-63 (1993).






























