« Prev Next »

The Evolution of Egg Color Patterns
Why do bird eggs range in color from uniformly white to brightly colored and/or densely speckled (maculated; see Banner photo)? Based on a comparison of eggshell patterns between different avian families, one of the most prevalent ecological factors responsible for the diversity of egg coloration is the interaction between brood parasites and their hosts (Kilner 2006). Obligate brood parasitic birds lay their eggs in nests of other species, thereby imposing a cost on hosts to raise genetically unrelated young (Davies 2000). Egg coloration and maculation play important roles in whether hosts accept or reject the fitness costs imposed by parasitism. For example, the blackcap (Sylvia atricapilla) is a host of the parasitic common cuckoo (Cuculus canorus) in Europe and typically rejects all non-mimetic (dissimilar) eggs (Honza et al. 2004). By experimentally parasitizing blackcap nests with host-like mimetic eggs (using eggs of other blackcaps), egg rejection drops to 36% (Polacikova et al. 2007). Accurate rejection of foreign eggs even at seemingly low rates can still be an adaptive behavior because the host reduces its chances of spending time and energy raising overly needy and genetically unrelated offspring.
These and other cuckoo hosts appear to have evolved a simple rule of thumb to direct their behavior: "eject the egg unlike your own". But how does a bird know what its own eggs look like? Researchers have tackled this question by experimentally manipulating the appearance of the bird's own egg by dying one, more, or all eggs in the same brood (Figure 1). Such studies reveal that great reed warbler (A. arundinaceus) hosts rely on both color differences between eggs and learned memories of their own eggs to recognize and reject cuckoo eggs (Moskát et al. 2010).

Truly astounding, though, is that selection for visual cues of recognition has resulted in the evolution of extreme level of egg color mimicry of specific hosts by different parasitic cuckoos (Figure 2). Through the process of coevolutionary arms race, egg mimicry also has likely influenced the perceptual sensitivities of hosts and their abilities to correctly identify and reject foreign eggs from the nest. The perceptual acuity necessary to make a correct rejection invites direct investigation; researchers can experimentally parasitize the nests of hosts with eggs of varying degrees of similarity, in order to determine the thresholds in color and maculation at which hosts make decisions to reject dissimilar, and likely foreign, eggs from their nest.

Avian Color Perception
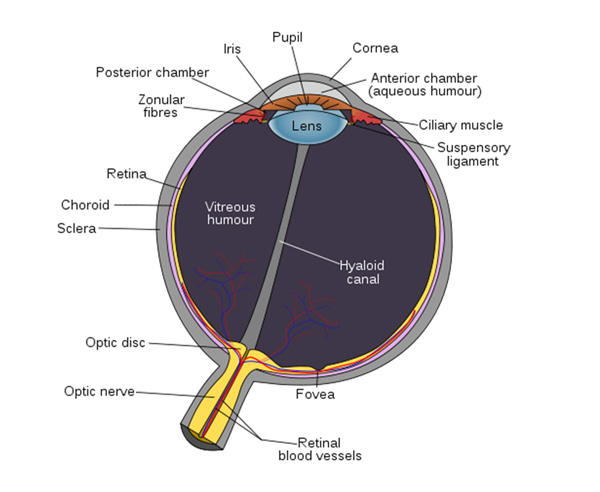
Before an experimenter can set out to manipulate egg colors, especially the ones hypothesized to be important for foreign egg rejection, it must be first established which colors that host species can see. The cone photoreceptors of the vertebrate retina (Figure 3) are responsible for color vision. The genes for opsins encode specific photopigments expressed in these cones, generating different combinations of proteins with maximal sensitivities to a particular wavelength of light (λmax). The cone cells of all color-sensitive vertebrates express opsins. The number of different opsins that an individual possesses is related to the number of colors to which it is sensitive.
Avian retinas differ from those of mammals in many ways, notably in the number of cone types that they possess. Unlike mammals, which typically have only two or three different cone types (Figure 4), bird species possess 4 distinct single cone types in their retinas, making them tetrachromatic (Figure 5; Hunt et al. 2009). Tetrachromats are theoretically able to see twice as many colors as trichromats (e.g., humans). For example, two eggs might appear indistinguishable to us, but a bird might see them displaying two distinct colors. This has direct implications for scientific investigations of avian perception — how can we manipulate egg colors when birds themselves may be more sensitive than we are to subtle differences in color?
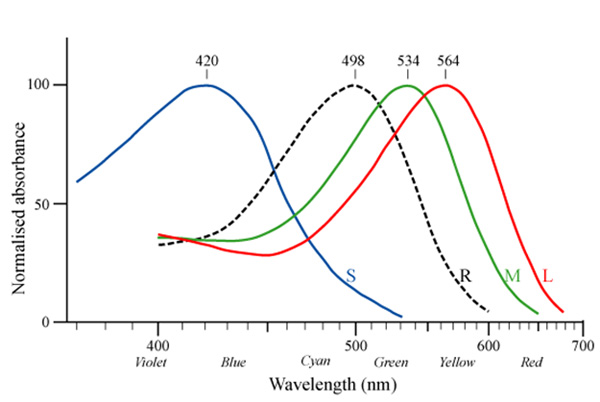
Some avian lineages, including many passerines, are able to see light in the ultraviolet (UV) range, which humans cannot see (Hart 2001). The hosts of brood-parasitic cuckoos in Europe and brown-headed cowbirds (Molothrus ater) in North America are passerines, implying that they might be able to perceive cryptic (to human) UV differences between their own and parasitic eggs to discriminate and reject parasitic eggs. One of the types of opsins (SWS1) is sensitive to the shortest wavelengths of light, and is found in all vertebrate classes (Hazel et al. 2006). In humans and many bird species, the SWS1 opsin is expressed in cones that respond maximally to violet light (such species are termed violet-sensitive, VS). In some passerine species, however, the SWS1 opsin gene codes for a photoreceptor with a λmax that crosses into the UV portion of the light spectrum (Figure 6). Species with this type of SWS1 cone are UV-sensitive (UVS; Hart 2001; Ödeen & Håstad 2003; Hunt et al. 2009).
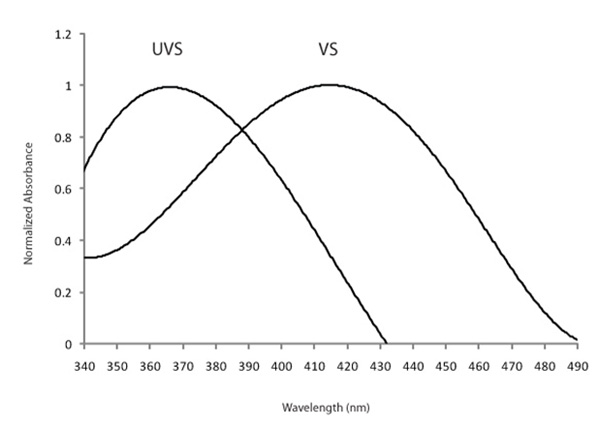
A UV-sensitive SWS1 is apparently ancestral among vertebrates, but was subsequently lost in primates and birds (Yokoyama 2000; Jacobs & Rowe 2004; this is a great review for those interested in the evolution of color vision in vertebrates). Among birds, however, UVS has re-evolved independently at least 4 times via a shift in SWS1 sensitivity (Hunt et al. 2009). UV-sensitivity in turn can serve a number of adaptive behavioral and ecological functions, including sexual displays, predator/prey detection, intraspecific communication to avoid detection by VS predators (Håstad et al. 2005), and defense mechanisms against egg mimicry in brood parasitism (Honza et al. 2007; Underwood & Sealy 2008). These two latter studies demonstrate that the UV-reflectance of eggs differs between host and parasite, suggesting that hosts can use UV-only visible patterns to discriminate between their own and foreign eggs. Whether UV-sensitivity evolved as a response to brood parasitism or was already available for hosts to utilize at the onset of their evolutionary history with brood parasitism, remains still unknown (Underwood & Sealy 2008).
Regarding other ecological contexts, eggs of cavity-nesting species tend to have higher UV-reflectance than eggs of open cup nesters (Aviles et al. 2006), providing further evidence that UV light can both be seen and be informative for parental birds' behavioral decisions. Accordingly, cavity-nesting spotless starlings (Sturnus unicolor) are more likely to accept experimental eggs placed just outside the nest cup within the cavity (by pulling them them into the nest) with high UV-reflectance than eggs with low UV-reflectance.
But how can we know whether the SWS1 opsin of a particular bird species will be maximally sensitive to UV or violet wavelengths of light? Much of our knowledge of the avian sensory world now derives from physiological and molecular techniques which describe the sensitivities of opsins present in the eye. The traditional method of microspectrophotometry allowed researchers to determine the λmax of any photoreceptor by transmitting light through it and measuring which wavelengths are absorbed (Govardovskii et al. 2000). More recently, DNA sequencing of the SWS1 opsin gene has allowed researchers to assign VS/UVS states in a more cost-effective and non-lethal manner, relevant for large scale comparative studies (Ödeen & Håstad 2003), including work with bird species of conservation concern for which invasive studies cannot be done (Igic et al. 2010).
The molecular machinery of the SWS1 photoreceptor requires only one amino acid substitution in a select few sites of the protein's amino-acid chain to change a VS species or individual to a UVS species or individual (Yokoyama et al. 2000). Genetic sequencing of the SWS1 opsin gene is now regarded as an accurate, reliable and economical alternative to microspectrophotometry (but see Smith et al. 2002).
Perceptual Modeling of the Avian Visual System
Integrative research spanning the fields of molecular genetics, physical light reflectance measurements, and behavioral experiments, has allowed researchers to quantify color patterns as birds would see and use them (Vorobyev & Osorio 1998; Endler & Mielke 2005). To interpret physiological and genetic data, however, requires perceptual models which are mathematical representations of what a bird can see, based on a number of different parameters, including the amount of light that reaches the retina and the relative abundance and type of photoreceptors present in that particular species' eyes. Using physiological data generated from genetic sequencing of the opsin genes (Ödeen & Håstad 2003), researchers can now produce reasonably accurate models of avian visual perception and its behavioral implications in egg rejection decisions (Cassey et al. 2008). Typically, the light reflectance of surfaces of interest, such as eggshells, is measured with a spectrophotometer and the resulting relative light reflectance data are then filtered through the perceptual model's equations to assess whether a species in question can see differences between particular light reflectance patterns, or colors.
Color Vision Links Sensory Ecology with Behavioral Decisions
References and Recommended Reading
Aviles, J. M., Soler, J. J. & Pérez-Contreras, T. Dark nests and egg colour in birds: a possible functional role of ultraviolet reflectance in egg detectability. Proceedings of the Royal Society of London B 273, 2821–2829 (2006).
Cassey, P., Honza, M., Grim, T. & Hauber, M. E. The modeling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biology Letters 4, 515–517 (2008).
Cassey, P. et al. Are avian eggshell colours effective intraspecific communication signals in the Muscicapoidea? A perceptual modelling approach. Ibis 151, 689–698 (2009).
Cassey, P. et al. Variability in avian eggshell colour: a comparative study of museum eggshells. PLoS ONE 5, e1 2054 (2010).
Davies, N. B. Cuckoos, Cowbirds, and Other Cheats. London: Poyser, 2000.
Endler, J. A. & Mielke, P. W. Comparing entire colour patterns as birds see them. Biological Journal of the Linnaean Society 86, 405-431 (2005).
Govardovskii, V. I., Fyhrquist, N., Reuter, T., Kuzmin, D. G. & Donner, K. In search of the visual pigment template. Visual Neuroscience 17, 509–528 (2000).
Hart, N. S. The visual ecology of avian photoreceptors. Progress in Retinal and Eye Research 20, 675–703 (2001).
Håstad, O., Victorsson, J. & Ödeen, A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proceedings of the National Academy of Sciences USA 102, 6391–6394 (2005).
Hill, G. E. & McGraw, K. J. Bird Coloration. Mechanisms and Measurements, vol. 1. Cambridge, MA: Harvard University Press, 2006.
Honza, M. et al. Are blackcaps current winners in the evolutionary struggle against the common cuckoo? Journal of Ethology 22, 175–180 (2004).
Honza, M., Polaciková, L. & Prochazka, P. Ultraviolet and green parts of the colour spectrum affect egg rejection in the song thrush (Turdus philomelos). Biological Journal of the Linnean Society 92, 269–276 (2007).
Hubbard, J. K., Uy, J. A. C., Hauber, M. E., Hoekstra, H. E. & Safran, R. J. Vertebrate pigmentation: from underlying genes to adaptive function. Trends in Genetics 26, 231–239 (2010).
Hunt, D. M., Carvalho, L. S., Cowing, J. A. & Davies, W. L. Evolution and spectral tuning of visual pigments in birds and mammals. Philosophical Transactions of the Royal Society of London B 364, 2941–2955 (2009).
Igic, B., Cassey, P., Samas, P., Grim, T. & Hauber, M. E. Cigarette butts form a perceptually cryptic component of song thrush (Turdus philomelos) nests. Notornis 56, 134–138 (2009).
Igic, B. et al. Size dimorphism and avian-perceived sexual dichromatism in a New Zealand endemic bird, the whitehead Mohoua albicilla. Journal of Morphology 271, 697–704 (2010).
Jacobs, G. H. & Rowe, M. P. Evolution of vertebrate colour vision. Clinical and Experimental Optometry 87, 206–216 (2004).
Kilner, R. M. The evolution of egg colour and patterning in birds. Biological Reviews 81, 383–406 (2006).
Moskát, C., Bán, M., Székely, T., Komdeur, J., Lucassen, R. W. G., van Boheemen, L. A. & Hauber, M. E. Discordancy or template-based recognition? Dissecting the cognitive basis of foreign eggs in hosts of avian brood parasites. Journal of Experimental Biology 213, 1976–1983 (2010).
Ödeen, A. & Håstad, O. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Molecular Biology and Evolution 20, 855–861 (2003).
Polacikova, L., Honza, M., Prochazka, P., Topercer, J. & Stokke, B.G. Colour characteristics of the blunt egg pole: cues for recognition of parasitic eggs as revealed by reflectance spectrophotometry. Animal Behaviour 74, 419–427 (2007).
Smith, E. L., Greenwood, V. J. & Bennet, A. T. D. Ultraviolet colour perception in European starlings and Japanese quail. Journal of Experimental Biology 205, 3299–3306 (2002).
Underwood, T. J. & Sealy, S. G. UV reflectance of eggs of brown-headed cowbirds (Molothrus ater) and accepter and rejecter hosts. Journal of Ornithology 149, 313–321 (2008).
Van Hazel, I., Santini, F., Mϋller, J. & Chang, B. S. W. Short-wavelength sensitive opsin (SWS1) as a new marker for vertebrate phylogenetics. BMC Evolutionary Biology 6, 97 (2006).
Vorobyev, M. & Osorio, D. Receptor noise as a determinant of colour thresholds. Proceedings of the Royal Society of London B 265, 351–358 (1998).
Yokoyama, S. Molecular evolution of vertebrate visual pigments. Progress in Retinal and Eye Research 19, 385–420 (2000).
Yokoyama, S., Radlwimmer, F. B. & Blow, N. S. Ultraviolet pigments in birds evolved from violet pigments by a single amino acid change. Proceedings of the National Academy of Sciences USA 97, 7366–7371 (2000).