« Prev Next »
What Is Australopithecus?
The genus Australopithecus is a collection of hominin species that span the time period from 4.18 to about 2 million years ago. Australopiths were terrestrial bipedal ape-like animals that had large chewing teeth with thick enamel caps, but whose brains were only very slightly larger than those of great apes. They are the closest known relatives of our genus Homo, and we most likely evolved from a species that was part of this adaptive radiation. They are similar to the group of animals referred to as Paranthropus by some authors, or “robust” australopiths by others (see Constantino’s article), but have less extreme adaptations for powerful chewing.
Australopithecus was originally defined by Raymond Dart in 1925 on the basis of a small child’s skull from Taung, South Africa, that was 2-3 million years old. This fossil had an ape-sized brain but a short face with smaller front teeth, including a small, human-like canine tooth much smaller than the projecting ones of apes, and larger back teeth (molars) than those of apes. Perhaps most importantly, the place where the spinal cord exited the skull was positioned underneath it, showing that these creatures stood fully upright with their head positioned over their vertebral column. Dart named this fossil species Australopithecus africanus (Dart 1925). The Taung skull was the first evidence showing that walking upright on two feet and a different diet (as inferred from the teeth) were the adaptations that initially set hominins apart from apes, and that these changes long preceded great expansion of the brain and the many complex behaviors that accompany it. Since the discovery of the Taung specimen, many hundreds of specimens from roughly eight species of Australopithecus have been discovered in South Africa (A. africanus, A. sediba), eastern Africa (Ethiopia, Kenya, Tanzania; A. anamensis, A. afarensis, A. deyiremeda, Kenyanthropus platyops) and central Africa (Chad; A. bahrelghazali). The oldest and most primitive australopiths are found in eastern Africa, particularly Ethiopia and Kenya, with more derived australopiths appearing later in South Africa. The eastern African fossils are often fragmentary because their skeletons tend to be the remains of carnivore or geological activity, or both (for more on Taphonomy, see Dunsworth’s article). An advantage is that they are found in sedimentological deposits that can be dated using radioactive decay methodology that provides accurate age estimates for these fossils (for more see Peppe’s article). South African sites yield some very well-preserved fossils and associated skeletons, but the complexities of the formation of the cave assemblages in which they are found can lead to uncertainty in dating. However, it appears that Australopithecus spans more than 2 million years of time and occupied a variety of habitats.
Dart described Australopithecus as “intermediate between living anthropoids and man” (1925: 574), but no formal definition of the genus was given beyond that. Nearly a century later, we understand australopiths to be intermediate in time and in anatomy between earlier hominins (Ardipithecus, Orrorin, Sahelanthropus; See Su’s article)and Homo, and the genus Australopithecus is defined not only by similarities among its species, but also by differences from the groups which pre- and post-date it. Strictly speaking, Australopithecus is probably not a true genus, because some species may be more closely related to humans than others, and anthropologists are not certain about the precise relationships among them. No Australopithecus species except perhaps the “robust” species A. robustus has been proposed to share a unique sister relationship with A. africanus, and so technically the genus Australopithecus should only very confidently be applied to the first to be named ,A. africanus, or perhaps to both A. africanus and A. robustus. However, australopiths are more similar in their adaptations to each other than to later Homo, so until a wider consensus is reached about Australopithecus phylogeny, and since there is such a large literature using the genus name Australopithecus, the genus name is still used by most paleoanthropologists. Overall, this genus represents an adaptive radiation, or group of closely related species that evolved from a common ancestor and diversified.
Biology of Australopithecus
Over the years, the discovery of additional species has rounded out our understanding of the adaptations characterizing this genus, “grade,” or evolutionary stage of hominin. Australopiths were fully upright bipeds whose skeletons display evidence of a history of selection for travelling bipedally on the ground, and that had lost features seen in most primates that would have made them good tree-climbers, such as a grasping foot (Figure 1). Key lines of evidence for upright posture include an upright position of the skull and a spine with curvatures allowing vertical posture, a short, broad pelvis providing effective leverage for propulsion and balance over the two lower limbs, a femoral carrying angle and a tibia oriented orthogonally to the ankle joint, which together position the feet directly under the knees as in humans today, and stiff feet with longitudinal and transverse arches that lacked opposable (grasping) big toes (reviews in Aiello and Dean 1990; Kimbel and Delezene 2009; Latimer 1991; Stern 2000; Ward et al. 2011; Ward 2002). There are also footprints of Australopithecus at Laetoli, Tanzania, 3.66 Ma that clearly indicate bipedal locomotion.
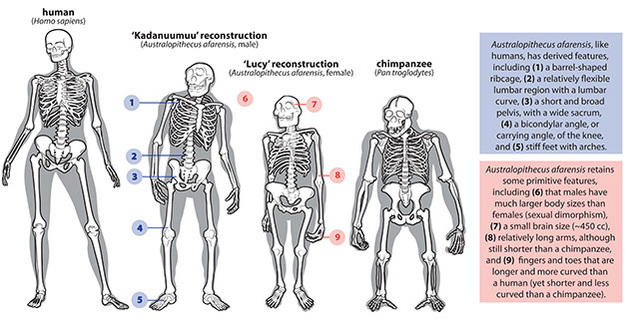
However, australopiths differed from Homo in having relatively large forelimbs relative to the length of their hindlimbs, longer and more curved hand and foot phalanges, evidence of a less sophisticated grip capability in the hand, perhaps one more functional lumbar vertebra, small vertebral bodies and hindlimb joints, and long and anteroposteriorly flattened femoral necks with small femoral heads (review in Stern 2000). These differences from Homo are often interpreted as reflecting continued reliance on arboreal climbing at least part-time. However, these differences may also be primitive features that were retained because they did not compromise bipedal locomotion, suggesting these morphologies were altered in Homo for some other reason, such as giving birth to larger babies, walking longer distances more efficiently, or carrying, manipulating and throwing objects with the hands.
Australopiths were roughly 1.2-1.5 m tall and probably weighed about 30-50 kg (McHenry 1992). Males were almost twice the size of females, a level of difference, or sexual dimorphism, greater than modern chimpanzees or humans but less than gorillas or orangutans (Hartwig-Scherer 1993; but see Lockwood 1999; Lockwood et al. 1996; McHenry 1992; Plavcan et al. 2005 but see; Reno et al. 2003; Reno et al. 2005). Their cranial capacity was 420-550 cc3, making their brains slightly larger for their body size than are those of modern apes (Falk et al. 2000; Holloway 1975; Tobias 1975).
Australopithecus species lack canine tooth size sexual dimorphism, and have canines much reduced in size compared with extant apes, only very slightly larger than those of females. This indicates that males were not using their teeth to bite each other during fighting for access to mates. However, because males were so much larger than females, they still probably competed heavily for access to females, which possibly signifies a novel means of male-male competition in these hominins. Australopith front teeth are smaller than those of extant apes, but the premolars and molars are expanded and thickly enameled (Figure 2). The jaws are robust, as is cranial evidence of the chewing apparatus, namely large cheekbones and generally well developed crests for attachment of neck and chewing muscles. The upper face is fairly vertical, but the lower face projects forward more than in humans (Figure 3). The face and dental anatomy suggests that australopiths were adapted to eating tough, hard-to-process foods such as tubers, nuts, seeds or roots, at least during times of food scarcity. Studies of isotopes within teeth, microscopic enamel wear, and of plant particles recovered from dental plaque all seem to confirm a tough diet (Henry et al. 2012).


Rare artifacts attributed to australopiths suggest that there is more to their behavior than what anatomical clues indicate. For quite some time paleoanthropologists have known about bone digging and probing tools from australopith sites in South Africa, but it is now apparent that at least some australopiths also made and used stone tools. Cutmarks have been found on animal bones at the 3.4 Ma site of Dikika, Ethiopia, which is a site where A. afarensis is known (McPherron et al. 2010). Furthermore, a novel stone tool industry predating even the Oldowan has been discovered at Lomekwi, Kenya, a site dated to 3.3 Ma (Harmand et al. 2015). The makers of these tools remains unknown. Kenyanthropus platyops is known from other localities at Lomekwi, as are a number of fossils not attributed to any taxon. No hominins are definitively associated with thee tools, but the Lomekwian archeology demonstrate that australopiths were making and using stone tools in the mid-Pliocene, and probably consuming meat when available.
A final, critical piece of australopith biology is their environmental preference. East and South African australopiths are not typically found alongside fauna that prefer fully open habitats (for instance, dry savannah grasslands). Most current evidence suggests that australopiths lived a range of heterogeneous environments, including dry and moist woodlands, scrublands, riverine forests, lake margins, and sometimes wooded grasslands (Behrensmeyer and Reed, 2013). In short, australopiths could tolerate a wide variety of habitats.
The Cast of Characters
In addition to the type species of Australopithecus, A. africanus, there are other species usually included within this genus. Australopithecus afarensis was named on the basis of fossils from Laetoli, Tanzania (Johanson et al. 1978), Hadar (Johanson et al. 1982a; Johanson and Taieb 1976; Johanson et al. 1982b), and the species now includes fossils from Maka (White et al. 2000), Dikika (Alemseged et al. 2006), and Woranso-Mille (Haile-Selassie et al., 2010a) in Ethiopia, and possibly Kenya as well (Brown et al. 2001; Kimbel 1988).
Australopithecus bahrelghazali was a species named for a jaw from Chad (Brunet et al. 1995), though it is possible that these are western representatives of A. afarensis (Alemseged et al. 2006; Kimbel and Delezene 2009; Leakey et al. 2001; Ward et al. 2001) and, if so, should be considered part of that species.
Australopithecus deyiremeda also dates to 3.5 Ma (Haile Selassie et al. 2015), and is known from only two mandibles, two partial maxillae and some teeth. Like A. afarensis, it has strong jaws and thickly enameled teeth, although appears to differ in other ways suggesting that it is indeed a distinct species.
Australopithecus afarensis is the most well known species, partly because of the famous “Lucy” skeleton (A.L. 288-1), and partly because it is known from most skeletal elements from male and female, young and old individuals. Much of our understanding of hominin origins is based on A. afarensis, because until recently it was the best known and earliest, with fossils known from at least 3.4 Ma (Haile-Selassie et al. 2010a; Kimbel et al. 1994) and footprints from 3.6 Ma (Leakey 1981).
However, a species that predates A. afarensis by almost a half a million years has been discovered from sites in Kenya (Kanapoi, 4.18 Ma (McDougall & Brown 2008); Allia Bay 3.9 Ma (Coffing et al. 1994)) and Ethiopia (Asa Issie 4.12 Ma (White et al., 2006); Fejej, 4.1 Ma (Fleagle et al. 1991)). Australopithecus anamensis and A. afarensis are almost certainly part of a single evolving lineage (Kimbel et al. 2006). Fossils intermediate in time and morphology from Woranso Mille, Ethiopia (3.7-8 Ma) reinforce this transition (Haile-Selassie et al. 2010b). Over time, there were changes in the geometry of the teeth, face and jaws that appear to reflect increasing chewing effectiveness for processing tough foods.
Australopithecus sediba (Berger et al. 2010), known from several skeletons from Malapa, South Africa, is argued to share a sister relationship with Homo based on a number of morphological characters, and if so would be more properly considered Homo. However, Australopithecus sediba is reported to have a reasonably well-developed masticatory system a very small brain for hominins, and perhaps more ape-like limb proportions than Homo. The phylogenetic relationship between A. sediba and Homo will be evaluated as more of the A. sediba material is published.
Australopithecus garhi is a species from 2.6 Ma, remarkable for its large molars (Asfaw et al., 1999), perhaps suggesting that this species actually belongs to the “robust” group of australopiths. A partial skeleton with Homo-like upper-arm to lower-arm proportions was discovered near the A. garhi craniodental remains but not in direct association, and so the postcranial anatomy of this species could be more advanced than other australopiths if the skeleton and skull belong to the same species. Also noteworthy is A. garhi’s proximity in time and space to what were the earliest cut-marked bones and stone tools (Asfaw et al., 1999), although the Dikika cutmarks and the Lomekwi stone tools (described above) suggest a much earlier beginning to the manufacture and use of stone tools than was previously appreciated.
The other species broadly considered within this group is Kenyanthropus platyops (Leakey et al. 2001; Spoor et al. 2010). This 3.3 Ma hominin from Lomekwi, Kenya, is known only from a crushed and distorted skull, and possibly also two partial jaws and some teeth, yet it displays morphologies not seen in contemporaneous hominins. It has small teeth and does not display the enhanced masticatory adaptations of robust and non-robust australopiths. Its discoverers placed it within its own genus, arguing that if a more narrow generic definition is accepted and the ‘robust’ species are attributed to Paranthropus, then placing the Lomekwi fossils in a new genus is appropriate since it is not allied with any known genus. If, however, an inclusive definition of Australopithecus is accepted that includes the robust species, then the Lomekwi sample could well be transferred to Australopithecus platyops. Both the strict rules of taxonomical nomenclature as well as hypotheses for evolutionary relationships are involved in this debate, as they are with many other scientific endeavors of this type.
In summary, Australopithecus represents a successful adaptive radiation of hominins that persisted for more than 2 million years of time - perhaps even longer than our genus, Homo, has done so far. Australopithecus species vary somewhat, but all australopiths generally have the same suite of anatomical features and they appear to be variations on the same adaptive theme. There were a variety of species that likely occupied a range of environmental niches. Whether any australopiths were in direct competition with one another or with Homo is uncertain. Although we cannot pinpoint the precise species that gave rise to Homo, it is almost certain that we arose from either one of these species, or another yet undiscovered species of Australopithecus. Certainly, the more we learn about our seemingly ‘bushy’ early family tree, the better we can understand our own origins.References and Recommended Reading
Aiello, L., & Dean, C. An Introduction to Human Evolutionary Anatomy. London: Academic Press (1990).
Alemseged, Z., Spoor, F., et al. A juvenile early hominin skeleton from Dikika, Ethiopia. Nature, 443, 296-301 (2006).
Asfaw, B., White, T., et al. Australopithecus garhi: a new species of early hominid from Ethiopia. Science, 284, 629-635 (1999).
Behrensmeyer, A., & Reed, K. Reconstructing the habitats of Australopithecus: paleoenvionments, site taphonomy, and faunas. The Paleobiology of Australopithecus. ED: Reed K.E., Fleagle J.G., Leakey R. E. New York: Springer. 41-60 (2013).
Berger, L. R., de Ruiter, D. J., et al. Australopithecus sediba: a new species of Homo-like australopith from South Africa. Science, 328, 195-204 (2010).
Brown, B., Brown, F. H., et al. New hominids from the Lake Turkana Basin, Kenya. Journal of Human Evolution, 41, 29-44 (2001).
Brunet, M., Beauvilain, A., et al. The first australopithecine 2,500 kilometres west of the Rift Valley (Chad). Nature, 378, 273-275 (1995).
Coffing, K., Feibel, C., et al. Four-million-year-old hominids from East Lake Turkana, Kenya. American Journal of Physical Anthropology, 93, 55-65 (1994).
Dart, R. A. Australopithecus africanus: the man-ape of South Africa. Nature, 115, 195 (1925).
Falk, D., Redmond, J. C., et al. Early hominid brain evolution: a new look at old endocasts. Journal of Human Evolution, 38, 695-717 (2000).
Fleagle, J. G., Rasmussen, D. T., et al. New hominid fossils from Fejej, Southern Ethiopia. J. Hum. Evol., 21, 145-152 (1991).
Haile-Selassie, Y., Latimer, B. M., et al. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proc Natl Acad Sci U S A, 107, 12121-12126 (2010a).
Haile-Selassie, Y., Saylor, B. Z., et al. New Hominid Fossils From Woranso-Mille (Central Afar, Ethiopia) and Taxonomy of Early Australopithecus. American Journal of Physical Anthropology, 141, 406-417 (2010b).
Haile-Selassie, Y., Gibert, L., et al. New species from Ethiopia further expands Middle Pliocene hominin diversity. Nature, 521, 483-488.
Harmand, S., Lewis, J. E., et al. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature, 521, 310-315.
Hartwig-Scherer, S. Body weight prediction in early fossil hominids: towards a taxon-"independent" approach. American Journal of Physical Anthropology, 92, 17-36 (1993).
Henry, A. G., Ungar, P. S., et al. The diet of Australopithecus sediba. Nature, 487, 90-93 (2012).
Holloway, R. L. (1975) Early hominid endocasts: volumes, morphology, and significance for hominid evolution. In Tuttle, R. H. (Ed.), Primate Functional Morphology and Evolution. Paris: Mouton.
Johanson, D. C., Lovejoy, C. O., et al. Morphology of the Pliocene partial hominid skeleton (A. L. 288-1) from the Hadar formation, Ethiopia. American Journal of Physical Anthropology, 57, 403-452 (1982a).
Johanson, D. C., & Taieb, M. Pliocene hominid discoveries in Hadar, Ethiopia. Nature, 260, 293-297 (1976).
Johanson, D. C., Taieb, M., et al. Pliocene hominids from the Hadar formation, Ethiopia (1973-1977): stratigraphic, chronologic and paleoenvironmental contexts, with notes on hominid morphology and systematics. American Journal of Physical Anthropology, 57, 373-402 (1982b).
Johanson, D. C., White, T. D., et al. A new species of the genus Australopithecus (Primates: Hominidae) from the Pliocene of eastern Africa. Kirtlandia, 28, 1-14 (1978).
Kimbel, W. H. Identification of a partial cranium of Australopithecus afarensis from Koobi Fora Formation, Kenya. Journal of Human Evolution, 17, 647-656 (1988).
Kimbel, W. H., & Delezene, L. K. "Lucy" redux: a review of research on Australopithecus afarensis. Am J Phys Anthropol, 140 Suppl 49, 2-48 (2009).
Kimbel, W. H., Johanson, D. C., et al. The first skull and other new discoveries of Australopithecus afarensis at Hadar, Ethiopia. Nature, 368, 449-451 (1994).
Kimbel, W. H., Lockwood, C. A., et al. Was Australopithecus anamensis ancestral to A. afarensis? A case of anagenesis in the hominin fossil record. J Hum Evol, 51, 134-152 (2006).
Latimer, B. (1991) Locomotor adaptations in Australopithecus afarensis: the issue of arboreality. In Coppens, Y. & Senut, B. (Eds.), Origine(s) de la Bipédie chez les Hominidés (pp. 169-176). Paris: Centre National de la Recherche Scientifique.
Leakey, M. Discoveries at Laetoli in northern Tanzania. Proceedings. Geologists Association., 92, 81-86 (1981).
Leakey, M. G., Spoor, F., et al. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature, 410, 433-440 (2001).
Lockwood, C. A. Sexual dimorphism in the face of Australopithecus africanus. American Journal of Physical Anthropology, 108, 97-127 (1999).
Lockwood, C. A., Richmond, B. G., et al. Randomization procedures and sexual dimorphism in Australopithecus afarensis. Journal of Human Evolution, 31, 537-548 (1996).
McDougall, I., & Brown, F. Geochronology of the pre-KBS Tuff sequence, Omo Group, Turkana Basin. Journal of the Geological Society, 165, 549-562 (2008).
McHenry, H. M. Body size and proportions in early hominids. American Journal of Physical Anthropology, 87, 407-431 (1992).
McPherron, S. P., Alemseged, Z., et al. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ato at Dikika, Ethiopia. Nature, 466, 857-860.
Plavcan, J. M., Lockwood, C. A., et al. Sexual dimorphism in Australopithecus afarensis revisited: how strong is the case for a human-like pattern of dimorphism? Journal of Human Evolution, 48, 313-320 (2005).
Reno, P. L., Meindl, R. S., et al. Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc Natl Acad Sci U S A, 100, 9404-9409 (2003).
Reno, P. L., Meindl, R. S., et al. The case is unchanged and remains robust: Australopithecus afarensis exhibits only moderate skeletal dimorphism. Journal of Human Evolution, 49, 279-288 (2005).
Spoor, F., Leakey, M. G., et al. Hominin diversity in the Middle Pliocene of eastern Africa: the maxilla of KNM-WT 40000. Philosophical Transactions B, 365, 3377-3388 (2010).
Stern, J. T. Climbing to the top: a personal memoir of Australopithecus afarensis. Evolutionary Anthropology, 9, 113-133 (2000).
Tobias, P. V. (1975) Brain evolution in the Hominoidea. In Tuttle, R. H. (Ed.), Primate Functional Morphology and Evolution. Paris: Mouton.
Ward, C., Kimbel, W. H., et al. Complete Fourth Metatarsal and Arches in the Foot of Australopithecus afarensis. Science, 331, 750-753 (2011).
Ward, C. V. Interpreting the posture and locomotion of Australopithecus afarensis: where do we stand? American Journal of Physical Anthropology, Suppl 35, 185-215 (2002).
Ward, C. V., Leakey, M. G., et al. Morphology of Australopithecus anamensis from Kanapoi and Allia Bay, Kenya. Journal of Human Evolution, 41, 255-368 (2001).
White, T. D., Suwa, G., et al. Jaws and teeth of Australopithecus afarensis from Maka, Middle Awash, Ethiopia. American Journal of Physical Anthropology, 111, 45-68 (2000).
White, T. D., WoldeGabriel, G., et al. Asa Issie, Aramis and the origin of Australopithecus. Nature, 440, 883-889 (2006).






























