« Prev Next »

Eusocial animals share the following four characteristics: adults live in groups, cooperative care of juveniles (individuals care for brood that is not their own), reproductive division of labor (not all individuals get to reproduce), and overlap of generations (Wilson 1971). Whereas primitively eusocial organisms show no morphological difference between reproductives and non-reproductives, advanced eusocial organisms may have different morphologies for reproductive and non-reproductive individuals and even specialization within the non-reproductives (e.g., soldier and worker castes in the army ant Eciton burchelli).
Other types of social interactions include subsociality, wherein there is social behavior between parents and offspring (e.g., birds, Halictine bees; Plateaux-Quénu 2008); and parasociality, wherein there is social behavior among members of the same generation (e.g., most bees).
Which Animals are Eusocial?
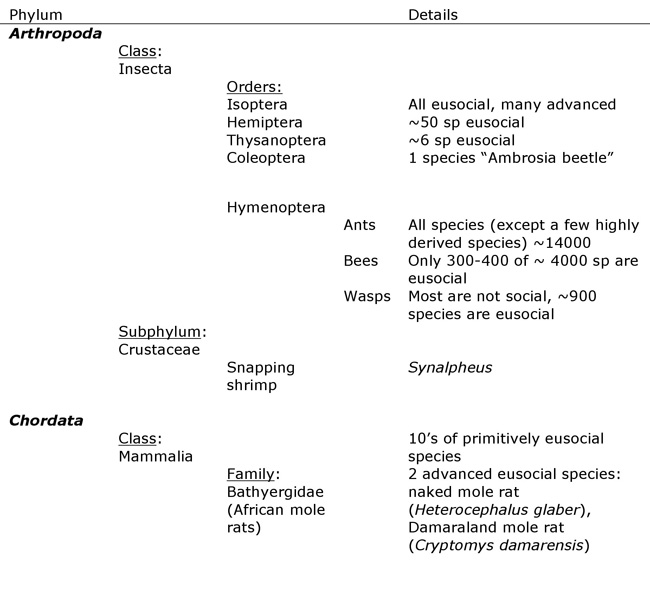
Most eusocial animals are found in the phylum Arthropoda, but a few are found in the phylum Chordata (Table 1).
The order Hymenoptera is the largest and most well-known animal group with eusocial species (see Figures 1–3). Most hymenoptera are not eusocial, but this social system has arisen multiple times within the group. It is found in some bees (Apidae) and wasps (Ross & Matthews 1991) — with separate origins of eusociality in Sphecidae and Vespidae — and all ants (Formicidae, which are most closely related to Vespidae; Hölldobler & Wilson 1990). Hymenoptera have a haplodiploid sex determination system (whereby females arise from fertilized diploid eggs and males arise from unfertilized haploid eggs), which may contribute to kin selection, favoring altruistic behavior in this group.

Termites can be considered to be highly evolved social cockroaches which live in their food (rotting wood; Thorne 1997). They depend on a complex mutualism with cellulose digesting protozoans and bacteria, and young individuals acquire these symbionts via anal trophallaxis. Within a colony, there is caste differentiation: there is a queen and king, the sole reproducing individuals, and there are soldiers and workers, which are different stages of pluripotent larvae of both sexes. Termites are diploid and perform complex social behaviors including nest construction (Ladley & Bullock 2005) and territorial defense (Adams 1987).
The more recently discovered eusocial organisms include a few species of shrimp, aphids, and thrips. There are at least two separate origins of eusociality within the Synalpheus shrimps (Duffy et al. 2000). These marine shrimp live in groups of several hundred closely related diploid individuals as internal parasites on tropical sponges. The host sponges have a heterogeneous distribution, and this may have contributed to the evolution of eusociality within this group as dispersal to establish new colonies is riskier than remaining within the natal nest.

Thrips are small haplodiploid insects in the order Thysanoptera (Crespi et al. 1992). Of about 5,000 species, about 300 form nests in plants called galls, feeding on plant tissue within. Of these, there are six species which can be classified as eusocial: they have morphologically different soldier castes which defend the galls from kleptoparasites.
Social aphids, like thrips, live in galls or hollowed stems of plants, feeding from the plant tissue (Stern 1994, Aoki & Imai 2005). These tiny hemipterans may have complex life cycles — they are diploid, but can reproduce parthenogenetically — and there have been several species described that have robust soldier morphs (Stern & Foster 1996).

There are at least two species of vertebrates that could be considered eusocial, the naked mole rat and the Damaraland mole rat (Burda et al. 2000). Both species are diploid, highly inbred and live in harsh deserts with patchy food resources. Most individuals help to raise siblings or close relatives that are born to a single reproductive female (the queen). Outbreeding results from the generation of dispersive morphs — individuals that have inbreeding avoidance and large fat stores — from the natal nest to start a new colony (O'Riain et al. 1996).
Advantages to Living in Groups
Eusocial organisms live in groups, and are subject to both the benefits and costs of group living. Living in a group may confer benefits on individuals in several ways. First, groups may form as defense against predation, forming a "selfish herd" in which each individual has a lower chance of being killed (Hamilton 1971). Second, larger groups in many species gain advantages against competitors, such as in colonies of the ant Azteca trigona (Adams 1994).
Many eusocial organisms have strategic advantage when acquiring food in groups (e.g., raiding army ants; Solé et al. 2000). Where there are limited nest sites or resources are patchy, the benefits to staying within the natal group include reducing dispersal risk and the possibility of inheriting the natal nest. This is a contributing factor in naked mole rat societies (Jacobs & Jarvis 1996) and in insect societies with totipotent workers such as primitive ants (Molet et al. 2005) and wasps (Monnin et al. 2009).
There can be costs to living in social groups, and these must ultimately be balanced by fitness advantages. Disadvantages include increased competition for resources between individuals, increased transmittance of parasites and diseases within groups, and easy detection of the group by predators and parasites.
How did Eusociality Evolve?
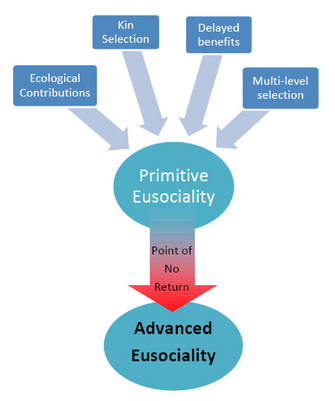
Giving up one's reproductive potential is contrary to the basic premise of natural selection (to survive and reproduce). Even Darwin (1859) commented on the challenge of understanding eusociality as "one special difficulty, which at first appeared to me insuperable, and actually fatal to the whole theory. I allude to the neuters or sterile females in insect communities: for these neuters often differ widely in instinct and in structure from both the males and fertile females, and yet, from being sterile, they cannot propagate their kind." Darwin goes on to argue that "This difficulty, though appearing insuperable, is lessened, or, as I believe, disappears, when it is remembered that selection may be applied to the family, as well as to the individual. . ." There have been several hypotheses proposed for the evolution of worker-like behavior. It is important to note that they are not mutually exclusive — each may play a different role in the evolution of eusociality in different groups (Figure 4). Evolutionary biologists trace the origins of eusociality through a pathway that starts with solitary organisms acquiring benefits to group behavior, eventually leading to a "point of no return" (Wilson & Hölldobler 2005) wherein certain individuals no longer have the physical ability to reproduce and only gain evolutionary fitness indirectly. In addition, it is important to note that the selective forces at work during the inception of eusocial behavior may be different from those that maintain advanced eusocial colonies (Hölldobler & Wilson 2009). What follows is a brief description of major contributing factors during the inception of eusocial behavior.
Kin Selection
There are two routes by which a gene can promote copies of itself in future generations: directly, through producing offspring, and indirectly, through the reproduction of close relatives. The sum of direct and indirect reproductive gains is known as inclusive fitness. As a result, it is possible for selection of eusociality over solitary behavior if indirect fitness levels exceed direct fitness.
An altruistic act is one which benefits a recipient at a cost to the performer of the act. Hamilton's rule (Hamilton 1964) states that altruism is favored if
r > C/B
where B is the benefit to recipient of altruistic act in terms of lifetime reproductive success and C is the cost (decrease in lifetime reproductive success). The coefficient of relatedness, r, ranges from 0 to 1 and is the proportion of alleles shared between two individuals identical by descent. Eusociality depends on high levels of altruism within groups as individuals increase the fitness of others at a cost to themselves (e.g., by helping care for brood, defending nests, sharing food resources, not investing in their own offspring).
There are two well-known mechanisms by which r can be elevated: haplodiploid sex determination and inbreeding. While is it not required for eusociality to evolve (naked mole rats and termites are diplodiploid), haplodiploidy may help to explain why eusociality has arisen multiple times within the Hymenoptera. In haplodiploid organisms, the relatedness between full sibling sisters (r = 0.75) is greater than between a mother and her offspring (r = 0.5), thus weighting Hamilton's rule in favor of raising sisters rather than offspring.
Inbreeding, or mating between close relatives, results in offspring which share a larger proportion of alleles, thus increasing r. This is common in organisms which don't disperse very long distances from the natal nest or are prone to mating with siblings (e.g., termites and wild naked mole rats; Thorne et al. 1999, Ciszek 2000).
Delayed Benefits
An intermediate step toward eusociality may have "hopeful reproductives" — that, workers which have the option to stay and help or to go out start their own nest. The decision can depend on hierarchical position within the group, territory, or food resources and other environmental conditions. Florida scrub jay offspring are known to stay at the natal nest (Breininger et al. 2010), helping to raise siblings (thus increasing their inclusive fitness) until there is an opportunity to replace their parents. Primitively eusocial wasp colonies, such as Polistes, are commonly inherited by dominant workers on the death of a queen (Monnin & Ratnieks 1999).
Multi-level Selection
Natural selection can occur at the level of the individual, family (related individuals, known as kin), or group (non-related individuals). This is known as multi-level selection. For eusocial organisms, the traits (phenotype) of the colony interact with the environment to determine colony-level fitness and can be described with models of multi-level or trait-group selection. Whether models of multi-level selection or inclusive fitness models are the best approach to explore the origin and maintenance of eusociality remains a strong point of debate (Gardener & Grafen 2009, Wilson & Wilson 2007, Leigh 2009, Nowak et al. 2010), especially since there is limited empirical evidence for inclusive fitness in groups (Seeley 1997). Robin Owen (1989) succinctly writes: "Ultimately, the unfolding of the evolutionary process entails selection on the genotypes of the founding female of the colony and her mates, operating through colony traits determines by the genotypes of the worker offspring they produce".
Ecological and Life History Contributions
Nesting behavior has been described as a possible prerequisite for the development of eusociality, in large part since it creates situations conducive to cooperative brood care (Anderson 1984). Where nest founding is dangerous or there are limited territories or spaces, "fortress defenders" can cooperate to defend this valuable resource (Queller & Strassmann 1998). In the case of termites, thrips, shrimp, and aphids, the protected nest is also the location of food in a patchy environment. Parental care can also be an important life history component. One path to eusociality in Hymenoptera is thought to start with solitary females engaging in simultaneous progressive provisioning — rearing multiple larvae of different ages at the same time (Field 2005). Transitioning to eusocial behavior would then incorporate remaining offspring and provisioning siblings, followed by offspring withholding their own reproduction.
Trends in the Research of Eusocial Behavior
In 2006, the first eusocial insect genome sequence (Apis mellifera) was completed, beginning a new era of "sociogenomics" (Honeybee Genome Sequencing Consortium 2006, Robinson et al. 2005). Sequencing is underway for several species of ants (Smith et al. 2009), with the goal to be able to understand the genetic underpinning of behavior, longevity, sociality and communication.
Central to the concept of eusociality is division of labor. Advanced eusocial species take this further than simply task differences but have different morphological castes. The interplay between genetic and environmental contributions to caste is being unraveled in several species (Schwander et al. 2010), including the utterly bizarre situation in harvester ants. There is a species with genetically distinct mitochondrial lineages derived from hybrid genomes from two species, P. barbatus and P. rugosus (Helms Cahan & Keller 2003). Queens must mate with males of both lineages, since workers are produced from between-lineage matings and reproductives are from within-lineage matings (Suni et al. 2007).
Sometimes referred to as "swarm intelligence" (Bonabeau et al. 1999), the mechanisms of sensible collective decision-making by large groups of individuals (e.g., half a million individuals in an army ant colony; Franks et al. 1991) have appealed to an interdisciplinary audience, from traffic control (Shtovba 2005) to optimization problems (Bonabeau et al. 2002). Without central leadership, groups rely on self-organized processes (Seeley 2002) involving consensus-building to find nests (Pratt 2005, Seeley et al. 2006), allocate workers to food resources (Seeley 2006, Beckers et al. 1990), or make territorial decisions (Hölldobler & Lumsden 1980, Plowes & Adams 2005). Social insects provide excellent inspiration for the burgeoning field of biomimicry, wherein everything from task allocation (Krieger et al. 2000) to nest architecture (Turner et al. 2008) has been shaped by natural selection and can serve as inspiration to solve human problems (Holbrook et al. 2010).
References and Recommended Reading
Adams, E. S. Territory size and population limits in mangrove termites. Journal of Animal Ecology 56, 1069–1081 (1987).
———. Territory defense by the ant Azteca trigona: maintenance of an arboreal ant mosaic. Oecologia 97, 202–208 (1994).
Anderson, M. The evolution of eusociality. Annual Review of Ecology, Evolution and Systematics 15, 165–189 (1984).
Aoki, S. & Imai, M. Factors affecting the proportion of sterile soldiers in growing aphid colonies. Population Ecology 47, 127–136 (2005).
Beckers, R. et al. Collective decision making through food recruitment. Insectes Sociaux 37, 258–267 (1990).
Bonabeau, E. et al. Swarm Intelligence: From Natural to Artificial Systems. Oxford, UK: Oxford University Press, 1999.
Bonabeau, E. et al. Inspiration for optimization from social insect behaviour. Nature 406, 39–42 (2002).
Breininger, D. R. et al. A model-selection approach to predicting whether Florida scrub-jays delay breeding. Condor 112, 378–389 (2010).
Burda, H. et al. Are naked and common mole-rats eusocial, and if so, why? Behavioral Ecology and Sociobiology 47, 293–303 (2000).
Bygott, J. et al. Male lions in large coalitions gain reproductive advantages. Nature 282, 839–841 (1979).
Ciszek, D. New colony formation in the "highly inbred" eusocial naked mole-rat: outbreeding is preferred. Behavioral Ecology 11, 1–6 (2000).
Crespi, B. et al. Ecology and evolution of galling thrips and their allies. Annual Review of Entomology 42, 51–71 (1992).
Darwin, C. On the Origin of Species by Means of Natural Selection. London, UK: John Murray, 1859.
Duffy, J. et al. Multiple origins of eusociality among sponge-dwelling shrimps (Synalpheus). Evolution 54, 503–516 (2000).
Field, J. The evolution of progressive provisioning. Behavioral Ecology 16, 770–778 (2005).
Franks, N. R. et al. The blind leading the blind in army ant raid patterns: testing a model of self-organization (Hymenoptera: Formicidae). Journal of insect Behavior 4, 583–607 (1991).
Gardener, A. & Grafen, A. Capturing the superorganism: a formal theory of group adaptation. Journal of Evolutionary Biology 22, 659–671 (2009).
Hamilton, W. D. Genetical Evolution of Social Behavior I. Journal of Theoretical Biology 7, 1–16 (1964).
Hamilton, W. D. Geometry for the selfish herd. Journal of Theoretical Biology 32, 295–311 (1971).
Helms Cahan, S. & Keller, L. Complex hybrid origin of genetic caste determination in harvester ants. Nature 424, 306–309 (2003).
Holbrook, C. T. et al. Social insects inspire human design. Biology Letters 6, 431–433 (2010).
Hölldobler, B. & Lumsden, C. J. Territorial strategies in ants. Science 210, 732–739 (1980).
Hölldobler, B. & Wilson, E. O. The Ants. Cambridge, MA: Belknap Press of Harvard University Press, 1990.
———. The Superorganism. New York, NY: W.W. Norton & Company, 2009.
Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443, 931–949 (2006).
Jacobs, D. & Jarvis, J. No evidence for the work-conflict hypothesis in the eusocial naked mole-rat (Heterocephalus glaber). Behavioral Ecology and Sociobiology 39, 401–409 (1996).
Krieger, M. J. et al. Ant-like task allocation and recruitment in cooperative robots. Nature 406, 992–995 (2000).
Ladley, D. & Bullock, S. The role of logistic constraints on termite construction of chambers and tunnels. Journal of Theoretical Biology 234, 551–564 (2005).
Leigh, E. The group selection controversy. Journal of Evolutionary Biology 23, 6–19 (2009).
Molet, M. et al. Dominance hierarchies reduce the number of hopeful reproductives in polygynous queenless ants. Insectes Sociaux 52, 247–256 (2005).
Monnin, T. & Ratnieks, F. Reproduction versus work in queenless ants: when to join a hierarchy of hopeful reproductives? Behavioral Ecology and Sociobiology 46, 413–422 (1999).
Monnin, T., Cini, A. et al. No actual conflict over colony inheritance despite high potential conflict in the social wasp Polistes dominulus. Proceedings of the Royal Society B: Biological Sciences 276, 1593–1601 (2009).
Nowak, M.A., Tarnita, C. E., & Wilson, E.O. The evolution of eusociality. Nature 466, 1057–1062 (2010).
O'Riain, M. et al. A dispersive morph in the naked mole-rat. Nature 380, 619–621 (1996).
Owen, R. E. "The genetics of colony-level selection." In The Genetics of Social Evolution, eds. Breed, D. & Page, Jr., R. E. (Boulder, CO: Westview Press, 1989): 31–60.
Plateaux-Quénu, C. Subsociality in Halictine bees. Insectes Sociaux 55, 335–346 (2008).
Plowes, N. J. R. & Adams, E. S. An empirical test of Lanchester's square law: mortality during battles of the fire ant Solenopsis invicta. Proceedings of the Royal Society B 272, 1809–1814 (2005).
Pratt, S. C. Quorum sensing by encounter rates in the ant Temnothorax albipennis. Behavioral Ecology and Sociobiology 16, 488–496 (2005).
Queller, D. & Strassmann, J. Kin selection and social insects. BioScience. 48, 165–175 (1998).
Robinson, G. E. et al. Sociogenomics: social life in molecular terms. Nature Reviews Genetics 6, 257–270 (2005).
Ross, K. & Matthews, R. The Social Biology of Wasps. Ithaca, NY: Cornell University Press, 1991.
Schwander, T. et al. Nature versus nurture in social insect caste differentiation. Trends in Ecology and Evolution 25, 275–282 (2010).
Seeley, T. D. Collective decision-making in honey bees: how colonies choose among nectar sources. Behavioral Ecology and Sociobiology 28, 277–290 (1991).
Seeley, T. D. Honey bee colonies are group-level adaptive units. American Naturalist 150, 22–41 (1997).
———. When is self-organization used in biological systems? Biological Bulletin 202, 314–318 (2002).
Seeley, T. D. et al. Group decision making in honey bee swarms. American Scientist 94, 220–229 (2006).
Shtovba, S. Ant algorithms: theory and applications. Program Comput Soft+ 31, 167–178 (2005).
Smith, C. D. et al. Ant genomics: strength and diversity in numbers. Molecular Ecology 19, 31–35 (2009).
Solé, R. V. et al. Pattern formation and optimization in army ant raids. Artificial Life 6, 219–226 (2000).
Stern, D. L. A phylogenetic analysis of soldier evolution in the aphid family Hormaphididae. Proceedings of the Royal Society B 256, 203–209 (1994).
Stern, D. & Foster, W. The evolution of soldiers in aphids. Biological Reviews 71, 27–79 (1996).
Suni, S. S. et al. Male parentage in dependent-lineage populations of the harvester ant Pogonomyrmex barbatus. Molecular Ecology 16, 5149–5155 (2007).
Thorne, B. Evolution of eusociality in termites. Annual Review of Ecology, Evolution and Systematics 28, 27–54 (1997).
Thorne, B. L. et al. Reproductive dynamics and colony structure of subterranean termites of the genus Reticulitermes (Isoptera Rhinotermitidae): a review of the evidence from behavioral, ecological, and genetic studies. Ethology, Ecology and Evolution 11, 149–169 (1999).
Turner, J. S. & Soar, R. C. Beyond Biomimicry: what termites can tell us about realizing the living building. In Proceedings of the 1st International Conference on Industrialized, Integrated, Intelligent Construction (I3CON), eds. Hassen, T. & Ye, J. (Loughborough, UK: I3CON, 2008) 221–237.
Wilson, E. O. The Insect Societies. Cambridge, MA: Belknap Press of Harvard University Press, 1971.
Wilson, E. O. & Hölldobler, B. Eusociality: origin and consequences. Proceedings of the National Academy of Sciences USA 102, 13367–13371 (2005).
Wilson, D. S. & Wilson, E. O. Rethinking the theoretical foundation of sociobiology. The Quarterly Review of Biology 82, 327–348 (2007).