« Prev Next »
What is the source and composition of the aerosols?
Atmospheric aerosols are suspensions of liquid, solid, or mixed particles with highly variable chemical composition and size distribution (Putaud et al. 2010). Their variability is due to the numerous sources and varying formation mechanisms (Figure 1). Aerosol particles are either emitted directly to the atmosphere (primary aerosols) or produced in the atmosphere from precursor gases (secondary aerosols).
Primary aerosols consist of both inorganic and organic components. Inorganic primary aerosols are relatively large (often larger than 1 μm) and originate from sea spray, mineral dust, and volcanoes. These coarse aerosols have short atmospheric lifetimes, typically only a few days. Combustion processes, biomass burning, and plant/microbial materials are sources of carbonaceous aerosols, including both organic carbon (OC) and solid black carbon (BC). BC is the main anthropogenic light-absorbing constituent present in aerosols. Its main sources are the combustion of fossil fuels (such as gasoline, oil, and coal), wood, and other biomass. Primary BC and OC containing aerosols are generally smaller than 1 µm.

Secondary aerosol particles are produced in the atmosphere from precursor gases by condensation of vapours on pre-existing particles or by nucleation of new particles. A considerable fraction of the mass of secondary aerosols is formed through cloud processing (Ervens et al. 2011). Secondary aerosols are small; they range in size from a few nanometres up to 1 µm and have lifetimes of days to weeks. Secondary aerosols consist of mixtures of compounds; the main components are sulphate, nitrate, and OC. The main precursor gases are emitted from fossil fuel combustion, but fires and biogenic emissions of volatile organic compounds (VOCs) are also important. Occasionally volcanic eruptions result in huge amounts of primary and secondary aerosols both at the ground and in the stratosphere (Boulon et al. 2011).
The size and chemical composition of the particles evolve with time through coagulation, condensation, and chemical reactions. Particles may grow by uptake of water, a process that depends on chemical composition, particle size, and ambient relative humidity. The different particles have varying impacts in the atmosphere depending on composition, and the numerous sources and large range in size distributions further complicate a quantification of their effects. Both particle growth and the mixing of different particle types influence the climate effect of aerosols.
How are aerosols distributed globally?
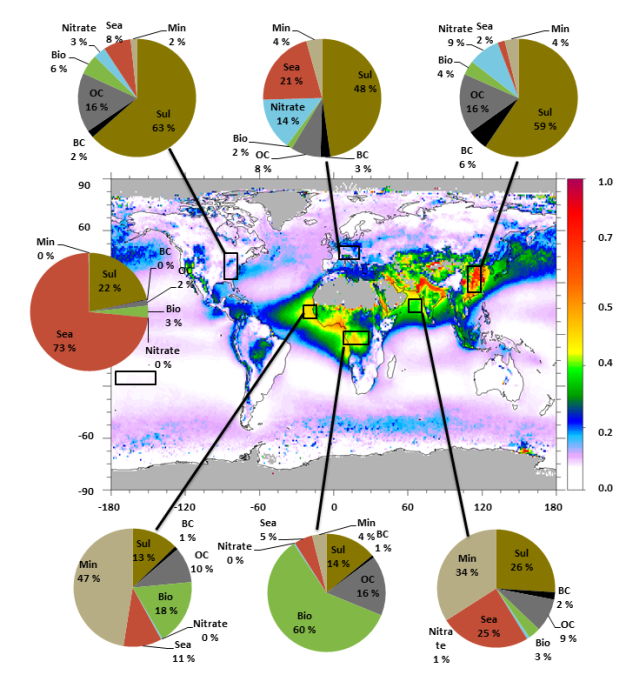
How do aerosols affect the climate?
All atmospheric aerosols scatter incoming solar radiation, and a few aerosol types can also absorb solar radiation. BC is the most important of the latter, but mineral dust and some OC components are also sunlight absorbers. Aerosols that mainly scatter solar radiation have a cooling effect, by enhancing the total reflected solar radiation from the Earth. Strongly absorbing aerosols have a warming effect. In the atmosphere, there is a mixture of scattering and absorbing aerosols, and their net effect on Earth's energy budget is dependent on surface and cloud characteristics. Scattering aerosols above a dark surface and absorbing aerosols above a bright surface are most efficient (see Figure 3a). Scattering (absorbing) aerosol above a bright (dark) surface are less efficient because the solar radiation is reflected (absorbed) anyway. Absorbing aerosols are particularly efficient when positioned above clouds, which are a main contributor to the total reflection of solar radiation back to space.
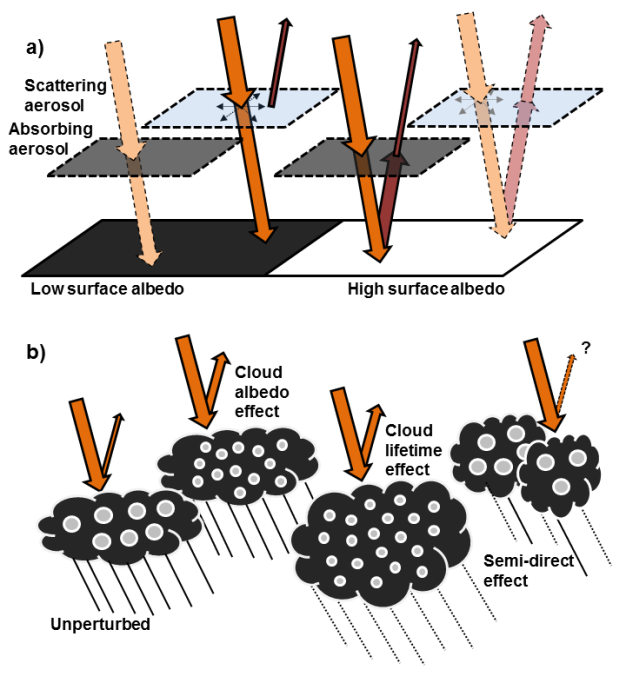
Aerosols are vital for cloud formation because a subset of them may serve as cloud condensation nuclei (CCN) and ice nuclei (IN). An increased amount of aerosols may increase the CCN number concentration and lead to more, but smaller, cloud droplets for fixed liquid water content. This increases the albedo of the cloud, resulting in enhanced reflection and a cooling effect, termed the cloud albedo effect (Twomey 1977; Figure 3b). Smaller drops require longer growth times to reach sizes at which they easily fall as precipitation. This effect, called the cloud lifetime effect, may enhance the cloud cover (see illustration in Figure 3b) and thus impose an additional cooling effect (Albrecht 1989). However, the life cycles of clouds are controlled by an intimate interplay between meteorology and aerosol-and-cloud microphysics, including complex feedback processes, and it has proven difficult to identify the traditional lifetime effect put forth by Albrecht (1989) in observational data sets.
Absorbing aerosols also have the potential to modify clouds properties, without directly acting as CCN and IN, by: (1) heating the air surrounding them while reducing the amount of solar radiation reaching the ground, which stabilizes the atmosphere and diminishes the convection and thus the potential for cloud formation, (2) increasing the atmospheric temperature, which reduces the relative humidity, inhibits cloud formation, and enhances evaporation of existing clouds. This is collectively termed the semi-direct aerosol effect (Hansen et al. 1997). The net effect is uncertain (see Figure 3b) and highly depends on the vertical profile of BC (Koch & Del Genio 2010).
In addition, BC and other absorbing aerosols deposited on snow or ice surfaces may reduce the surface albedo, leading to reduced reflectance of solar radiation, and hence a heating effect (Hansen & Nazarenko 2004).
Radiative forcing (RF) is often used to quantify and compare the potential climate impact of the various aerosol effects. RF is defined as a change in the Earth's radiation balance due to a perturbation of anthropogenic or natural origin.. The total aerosol forcing probability density function (PDF), in addition to individual aerosol components, indicating both the magnitudes and uncertainty of the effects, is shown in Figure 4a. The wider a PDF, the larger is the uncertainty. Combining all aerosol effects (blue dashed curve in Figure 4a) enhances the uncertainty compared to considering only the direct aerosol effect and cloud albedo effect.
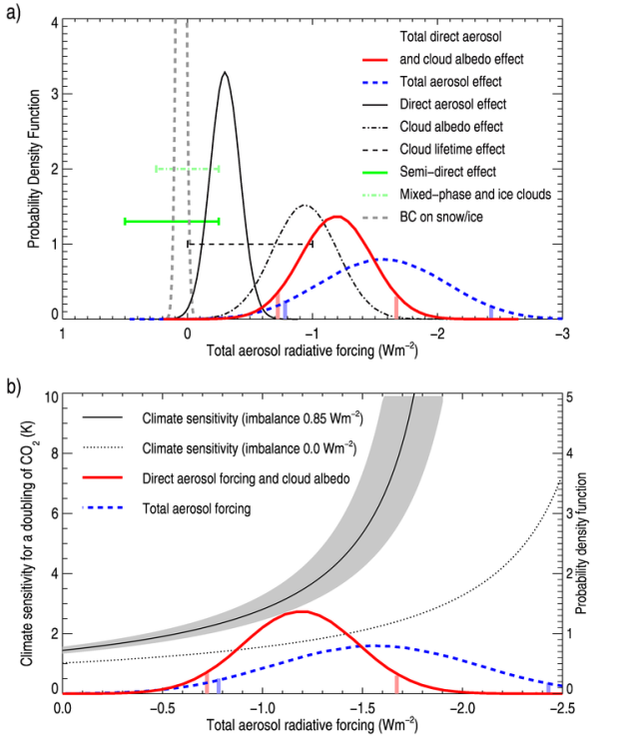
Why is the uncertainty in the aerosols important for predictions of climate sensitivity?
c d(ΔT)/dt = RF - ΔT/λ (1)
Here c is the heat capacity of the land-ocean-atmosphere system and λ is the climate sensitivity. At radiative equilibrium (d(ΔT)/dt = 0), Equation 1 reduces to ΔT = λRF. However, the Earth is not in radiative equilibrium, since less thermal radiation is currently emitted to space compared to what is absorbed of solar radiation (Hansen et al. 2005). This radiative imbalance causes the Earth to gradually warm, with global warming as a result (Trenberth & Fasullo 2010). The simple equation above has two key uncertainties. The observed surface temperature change is rather well determined over the industrial era, but the climate sensitivity and the total RF are both highly uncertain. The climate sensitivity is an essential parameter for prediction of future climate change. Quantifying the climate sensitivity for the doubling of CO2 has long been attempted by using global climate models or temperature records, but it still has a wide range of reported values (IPCC 2007, Knutti & Hegerl 2008). The total RF through the industrial era is also uncertain, mainly due to lack of quantification of the aerosol effects discussed above. The implication of this uncertainty in the aerosol RF for the quantification of the climate sensitivity can be illustrated as follows:

If we assume a total aerosol RF and a current energy imbalance, we can compute the resulting climate sensitivity using Equation 1 (Figure 4b). This can then be compared with the PDFs for the current aerosol RF to get an indication of the range in climate sensitivities allowed by the present knowledge (red and blue lines in figure 4b). A similar figure has previously been presented in Andreae et al. (2005). The allowed climate sensitivity ranges from about 2 to 8 Kelvin (K) for a doubling of CO2 using the known industrial age warming of around 0.8 K, the present best knowledge of RF from non-aerosol components, the 90% confidence interval of the total aerosol RF for the most certain effects, and radiative imbalance.
Has there been any progress in the understanding of the climate effect of aerosols?
An example of recent progress is reduced uncertainty in the estimate of the total direct aerosol effect. This estimate was made possible by advances that have occurred on both the modelling and the observational side, and was based on a combination of global aerosol models and observation based methods (mostly remotely sensed data). Initially, observational estimates of RF were up to three times stronger than model based calculations (Forster et al. 2007). Consistency between these two different approaches has subsequently been reached, and was found to arise from necessary and simplified assumptions of the pre-industrial aerosol composition in the observation-based method (Myhre 2009). Although the uncertainty in the total direct aerosol effect is reduced, it is still substantial compared to uncertainties associated with greenhouse gases. In addition the uncertainty in individual RF for several of the aerosol components, such as BC, OC, and nitrate, is large.
Similar to the early estimates of the direct aerosol effect, many of the first model estimates of the aerosol indirect effect only accounted for the effect of sulphate particles acting as CCN ( Kaufman & Chou 1993, Jones et al. 1994). Furthermore, they only included the influence of sulphate aerosols on cloud albedo, disregarding any effects on cloud lifetime and extent. With the realization that other aerosol species of anthropogenic origin could also form cloud droplets and that effects on cloud lifetime and extent were also possible, global climate models estimated the aerosol indirect effect to be stronger (e.g., Lohmann & Feichter 1997, Menon et al. 2002). Some even predicted this cooling effect to be comparable in magnitude to the warming greenhouse effect. Recent publications have later pointed to oversimplifications in model representation of clouds and how their lifetimes are affected by aerosols (e.g., Stevens & Feingold 2009). It is now acknowledged that aerosol effects on cloud lifetime will vary with the cloud type in question, and that complex feedback processes can sometimes complicate the ultimate cloud response to aerosol perturbations. Recent model studies have found that by forming ice in super-cooled liquid clouds, aerosols may in fact shorten cloud lifetime, because of the more efficient precipitation formation when cloud ice is present (e.g., Lohmann & Hoose 2009, Storelvmo et al. 2011). In summary, whether aerosols are acting as CCN or IN or are simply modifying atmospheric stability by absorbing solar radiation, there is still high uncertainty associated with their effect on cloud lifetime. This uncertainty reflects how challenging it is to represent aerosol-and-cloud processes that occur on microscopic scales in models that have resolutions of tens to hundreds of kilometres. Although much uncertainty remains, model and satellite estimates of the cloud albedo effect seem to converge on a negative RF that has about half the magnitude of the positive RF attributed to increasing CO2 concentrations.
Acknowledgements
References and Recommended Reading
Albrecht, B. A. Aerosols, cloud microphysics, and fractional cloudiness. Science 245, 1227-1230 (1989).
Andreae, M. O. et al. Strong present-day aerosol cooling implies a hot future. Nature 435, 1187-1190 (2005).
Andrews, E. et al. Climatology of aerosol radiative properties in the free troposphere. Atmospheric Research 102, 365-393 (2011).
Boucher, O. & Haywood, J. On summing the components of radiative forcing of climate change. Climate Dynamics 18, 297-302 (2001).
Boulon, J. et al. Observations of nucleation of new particles in a volcanic plume. Proceedings of the National Academy of Sciences (USA) 108, 12223-12226 (2011).
Charlson, R. J. et al. Perturbation of the Northern-Hemisphere radiative balance by backscattering from anthropogenic sulfate aerosols. Tellus Series a-Dynamic Meteorology and Oceanography 43, 152-163 (1991).
Ervens, B. et al. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): a review of laboratory, field and model studies. Atmospheric Chemistry and Physics 11, 11069-11102 (2011).
Forster, P. et al. Changes in Atmospheric Constituents and in Radiative Forcing, Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press, 2007.
Fullerton, D. G. et al. Biomass fuel use and indoor air pollution in homes in Malawi. Occupational and Environmental Medicine 66, 777-783 (2009).
Graber, E. R. & Rudich, Y. Atmospheric HULIS: How humic-like are they? A comprehensive and critical review. Atmospheric Chemistry and Physics 6, 729-753 (2006).
Hansen, J. & Nazarenko, L. Soot climate forcing via snow and ice albedos. Proceedings of the National Academy Of Sciences (USA) 101, 423-428 (2004).
Hansen, J. et al. Earth's energy imbalance: Confirmation and implications. Science 308, 1431-1435 (2005).
Hansen, J. et al. Radiative forcing and climate response. Journal of Geophysical Research-Atmospheres 102, 6831-6864 (1997).
Haywood, J. M. & Shine, K. P. The effect of anthropogenic sulfate and soot aerosol on the clear-sky planetary radiation budget. Geophysical Research Letters 22, 603-606 (1995).
Holben, B. N. et al. AERONET - A federated instrument network and data archive for aerosol characterization. Remote Sensing of Environment 66, 1-16 (1998).
IPCC. The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge, UK: Cambridge University Press, 2007
Isaksen, I. S. A. et al. Atmospheric composition change: Climate-chemistry interactions. Atmospheric Environment 43, 5138-5192 (2009).
Jacobson, M. Z. Global direct radiative forcing due to multicomponent anthropogenic and natural aerosols. Journal of Geophysical Research-Atmospheres 106, 1551-1568 (2001).
Jones, A. et al. A climate model study of indirect radiative forcing by anthropogenic sulfate aerosols. Nature 370, 450-453 (1994).
Kanakidou, M. et al. Organic aerosol and global climate modelling: A review. Atmospheric Chemistry and Physics 5, 1053-1123 (2005).
Kaufman, Y. J. & Chou, M. D. Model simulations of the competing climatic effects of SO2 and CO2. Journal of Climate 6, 1241-1252 (1993).
Knutti, R. & Hegerl, G. C. The equilibrium sensitivity of the Earth's temperature to radiation changes. Nature Geoscience 1, 735-743 (2008).
Koch, D. & Del Genio, A. D. Black carbon semi-direct effects on cloud cover: review and synthesis. Atmospheric Chemistry and Physics 10, 7685-7696 (2010).
Koch, D. et al. Distinguishing Aerosol Impacts on Climate over the Past Century. Journal of Climate 22, 2659-2677 (2009).
Liao, H. & Seinfeld, J. H. Global impacts of gas-phase chemistry-aerosol interactions on direct radiative forcing by anthropogenic aerosols and ozone. Journal of Geophysical Research-Atmospheres 110, D18208 (2005).
Lohmann, U. & Feichter, J. Impact of sulfate aerosols on albedo and lifetime of clouds: A sensitivity study with the ECHAM4 GCM. Journal of Geophysical Research-Atmospheres 102, 13685-13700 (1997).
Lohmann, U. & Hoose, C. Sensitivity studies of different aerosol indirect effects in mixed-phase clouds. Atmospheric Chemistry and Physics 9, 8917-8934 (2009).
Menon, S. et al. GCM Simulations of the aerosol indirect effect: Sensitivity to cloud parameterization and aerosol burden. Journal of the Atmospheric Sciences 59, 692-713 (2002).
Myhre, G. Consistency between satellite-derived and modeled estimates of the direct aerosol effect. Science 325, 187-190 (2009).
Myhre, G. et al. Modelled radiative forcing of the direct aerosol effect with multi-observation evaluation. Atmospheric Chemistry and Physics 9, 1365-1392 (2009).
Novakov, T. et al. Airborne measurements of carbonaceous aerosols on the East Coast of the United States. Journal of Geophysical Research-Atmospheres 102, 30023-30030 (1997).
Pósfai, M. et al. Soot and sulfate aerosol particles in the remote marine troposphere. Journal of Geophysical Research-Atmospheres 104, 21685-21693 (1999).
Putaud, J. P. et al. A European aerosol phenomenology-3: Physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across Europe. Atmospheric Environment 44, 1308-1320 (2010).
Quinn, P. K. & Bates, T. S. Regional aerosol properties: Comparisons of boundary layer measurements from ACE 1, ACE 2, aerosols99, INDOEX, ACE asia, TARFOX, and NEAQS. Journal of Geophysical Research-Atmospheres 110, D14202 (2005).
Ramanathan, V. et al. Indian Ocean Experiment: An integrated analysis of the climate forcing and effects of the great Indo-Asian haze. Journal of Geophysical Research-Atmospheres 106, 28371-28398 (2001).
Remer, L. A. et al. Global aerosol climatology from the MODIS satellite sensors. Journal of Geophysical Research-Atmospheres 113, D14s07 (2008).
Schulz, M. et al. Radiative forcing by aerosols as derived from the AeroCom present-day and pre-industrial simulations. Atmospheric Chemistry and Physics 6, 5225-5246 (2006).
Stevens, B. & Feingold, G. Untangling aerosol effects on clouds and precipitation in a buffered system. Nature 461, 607-613 (2009).
Storelvmo, T. et al. Global modeling of mixed-phase clouds: The albedo and lifetime effects of aerosols. Journal of Geophysical Research-Atmospheres 116, D05207 (2011).
Trenberth, K. E. & Fasullo, J. T. CLIMATE CHANGE Tracking Earth's Energy. Science 328, 316-317 (2010).
Twomey, S. Influence of pollution on shortwave albedo of clouds. Journal of Atmospheric Sciences 34, 1149-1152 (1977).
Zhang, Q. et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophysical Research Letters 34, L13801 (2007).































