Abstract
Cancer stem cells (CSCs), also termed “cancer-initiating cells” or “cancer progenitor cells,” which have the ability to self-renew, proliferate, and maintain the neoplastic clone, have recently been discovered in a wide variety of pediatric tumors. These CSCs are thought to be responsible for tumorigenesis and tumor maintenance, aggressiveness, and recurrence due to inherent resistance to current treatment modalities such as chemotherapy and radiation. Oncolytic virotherapy offers a novel, targeted approach for eradicating pediatric CSCs using mechanisms of cell killing that differ from conventional therapies. Moreover, oncolytic viruses have the ability to target specific features of CSCs such as cell-surface proteins, transcription factors, and the CSC microenvironment. Through genetic engineering, a wide variety of foreign genes may be expressed by oncolytic viruses to augment the oncolytic effect. We review the current data regarding the ability of several types of oncolytic viruses (herpes simplex virus-1, adenovirus, reovirus, Seneca Valley virus, vaccinia virus, Newcastle disease virus, myxoma virus, vesicular stomatitis virus) to target and kill both CSCs and tumor cells in pediatric tumors. We highlight advantages and limitations of each virus and potential ways in which next-generation engineered viruses may target resilient CSCs.
Similar content being viewed by others
Main
Cancer affects nearly 15 of every 100,000 children in the United States, and although survival rates have improved greatly over the past 30 y owing to cooperative trials and advances in surgical techniques, chemotherapy, and radiotherapy regimens, a significant subset of children, approximately 20%, succumb to their disease (1). Death can result from tumor progression or from ensuing toxicities caused by treatment. In the past, new treatment regimens have focused on increasing the dose of current therapies or combining multiple cytotoxic agents for patients with high-risk disease; however, current regimens already approach the upper limits of tolerability. Therefore, simply increasing the dose of current therapies or expanding treatment regimens with more cytotoxic agents is likely to worsen toxicities with minimal improvement in survival rates. The latest research has focused on determining which cells are responsible for tumor recurrence and finding ways in which these cells may be targeted in order to decrease toxicity and enhance quality of life and survival rates for children with cancer.
Recently, the cells thought to be responsible for tumorigenesis, tumor maintenance, aggressiveness, and recurrence have been identified in a number of pediatric malignancies (2). Termed “cancer stem cells” (CSCs), these malignant cells retain many of the capabilities of normal stem cells, including the ability to differentiate into multiple cell types, self-renew, proliferate, and maintain the neoplastic clone. Whether these are true stem cells is the subject of much debate; the cells may be in a further stage of differentiation than a true stem cell and therefore have also been called “cancer progenitor cells.” The fact that these cells can initiate tumors has led some to describe them as “cancer-initiating cells.” The term “CSCs” will be used throughout this review with the understanding that the actual nature of these cells is not entirely clear.
CSCs are thought to create and reside in a specialized microenvironment or niche where tumor and CSC regulation occurs through oxygen tension, cell-to-cell interactions, the extracellular matrix, and the balance of signals received through embryonic signaling pathways (3,4,5). Importantly, CSCs are characteristically resistant to traditional chemotherapy and radiotherapy and, consequently, are believed to be responsible for tumor recurrence (6,7). Mechanisms that pediatric CSCs use to resist current therapies include efficient DNA repair ability with preferential activation of DNA damage response, upregulation of antiapoptotic genes, differential expression and phosphorylation of various kinases, increased expression of ATP-binding cassette (ABC) transporters, and relative quiescence (2).
One of the main challenges for researchers is developing methods to identify and distinguish these cells from other tumor cells and normal cells in order to gain a better understanding of CSC biology and to develop novel targeted therapies to attack and kill CSCs. Current techniques to identify CSCs rely on an assortment of markers including cell-surface, nuclear, or cytoplasmic proteins; transcription factors; enzymes; and/or functional attributes (2). These unique features of CSCs, along with the niche in which they reside, offer potential strategies for targeting CSCs; novel approaches may direct an attack at CSC surface antigens, the niche, embryonic signaling and self-renewal pathways, angiogenesis, or mechanisms of resistance such as ABC transporters or DNA repair (2,8).
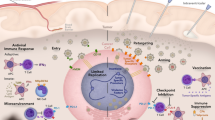
One such innovative, targeted therapy with preclinical efficacy in a variety of pediatric malignancies that may be well suited to eradicate resilient pediatric CSCs is oncolytic virotherapy, which kills tumor cells, releasing infectious virus to extend the therapy to neighboring tumor cells (9,10). Oncolytic viruses can be deadly to cancer cells and CSCs in three main ways: (i) viruses can directly target and attack tumor cells due to genetically engineered mutations that prevent viruses from either infecting or replicating in normal cells while permitting infection/replication in tumor cells; (ii) some viruses can be engineered to express therapeutic foreign gene products that either directly or indirectly result in cell death; or (iii) viruses that normally do not cause significant disease in humans may infect and kill tumor cells that contain altered signaling pathways or deficient interferon responses ( Figure 1 ). Oncolytic viruses use methods of cell killing that differ from traditional therapies and thus are able to elude the typical mechanisms that CSCs use to resist current chemotherapy and radiotherapy. Moreover, oncolytic viruses have the ability to target specific features of CSCs such as cell-surface proteins, transcription factors, and the CSC microenvironment. This review will focus on the current research regarding the ability of oncolytic viruses to target and kill CSCs in pediatric tumors and highlight potential ways in which next-generation viruses may target resilient CSCs. Viruses that have already entered clinical trials or have been used clinically in children include herpes simplex virus-1 (HSV), adenovirus, reovirus, Seneca Valley virus (SVV), vaccinia virus (VV), and Newcastle disease virus (NDV). Viruses that are being studied preclinically for possible use in children include myxoma virus (MYXV) and vesicular stomatitis virus (VSV). Table 1 provides a comparison of the benefits and limitations of the viruses to be discussed below.
Oncolytic viral therapy with or without gene therapy may be used to target cancer stem cells (CSCs). Virus can be delivered systemically or via direction injection into the tumor bed. Viral mutations (e.g., deletion of virulence genes) or nonhuman host range prevents a productive infection in normal cells but permits infection in CSCs. CSC-specific surface antigens may be targeted for viral entry. As viral replication ensues, foreign gene products are produced such as cytokines (e.g., interleukin-12), enzymes (e.g., chondroitinase), or other proteins (e.g., angiostatin). After host-cell lysis and release of foreign products, cytokines can result in an immune response (T cells (T), natural killer cells (NK), and macrophages (MΦ)) against CSC antigens in uninfected cells. Enzymes or inhibitory proteins can disrupt the CSC microenvironment. New viral particles can infect adjacent tumor cells.
Herpes Simplex Virus
HSV is a double-stranded, enveloped DNA cytolytic virus that has shown promise in treating a variety of pediatric malignancies including brain tumors, neuroblastoma, and sarcomas (11). Deletions or mutations of essential HSV-1 genes (e.g. γ134.5 “neurovirulence gene”) required for effective viral replication in normal cells but not cancer cells enable the virus to target malignant cells (12). Furthermore, a large portion (up to 30 kb) of the HSV genome is nonessential for the virus to replicate in cancer cells and, consequently, can be substituted with foreign DNA that can be used in several ways to enhance viral efficacy, such as by restoring the neurovirulence gene under control of a tumor-specific promoter to improve replication in tumor cells, by producing enzymes that disrupt and inhibit the tumor microenvironment, or by generating cytokines that can stimulate an immune response against the tumor. Importantly, clinically available antiviral agents (e.g., acyclovir, ganciclovir) are effective against mutant HSV in the unlikely event that the virus causes toxicity to normal cells.
HSV is a neurotropic virus, thus rendering neural malignancies like pediatric brain tumors and neuroblastomas, tumor types that have been reported to contain CSCs, ideal treatment targets (13,14,15,16,17). Two mutant viruses, G207 and HSV1716, have been used safely without any dose-limiting toxicities in adult patients with recurrent glioblastoma multiforme (GBM) ( Table 2 summarizes viruses included in the text) (18,19). G207 is a doubly deleted, genetically engineered HSV that was originally derived from the wild-type clinical isolate, HSV-1 (F). G207 HSV has had both copies of the γ134.5 gene deleted, combined with an insertional deletion of the UL39 gene encoding ICP6 (the heavy chain for ribonucleotide reductase). Insertion of the lacZ gene encoding β-galactosidase effectively disables expression of ribonucleotide reductase while providing a useful marker. HSV1716 was produced by deleting both copies of γ134.5 from wild-type HSV strain 17. Preclinical studies in neonatal mice and New World owl monkeys (Aotus nancymae) that are as sensitive to wild-type HSV-1 as human neonates suggest that engineered HSV will be safe in pediatric patients as well (20,21). The efficacy of mutant HSV has been demonstrated preclinically in pediatric gliomas, medulloblastomas, and neuroblastomas (22,23,24,25).
Recent research has examined the ability of mutant HSVs to kill CSCs from pediatric neural tumors ( Table 3 summarizes studies using oncolytic viruses to target pediatric CSCs). We showed that pediatric GBM xenograft D456MG contains CSCs marked by expression of CD133 (prominin-1), a transmembrane protein with uncertain biological function expressed on the surface of neuronal and hematopoietic stem cells and CSCs in a variety of malignancies (23). CSCs isolated from this xenograft were as sensitive as non-CSC tumor cells (CD133− cells) to several different γ134.5-deleted viruses including M002, an engineered HSV that expresses murine interleukin (IL)-12. IL-12 is an example of a cytokine added to elicit an immune response via activation of natural killer and T cells. The pediatric GBM D456MG was more sensitive to killing than several adult GBM xenografts tested (2,23). To increase tumor selectivity and enhance viral replication, Kambara et al. developed rQNestin34.5, which expresses ICP-34.5 under control of a synthetic nestin promoter (26). Nestin is an intermediate filament protein that is expressed in embryonic neuroglial cells and has been used as a CSC marker in a number of cancers including brain tumors and neuroblastoma. Mahller et al. used the rQNestin34.5 virus to infect and kill neuroblastoma CSCs (17). The virus prevented the CSCs from forming tumors in athymic nude mice, suggesting that the CSCs may be effectively targeted.
Another approach to targeting CSCs with engineered HSV is to disrupt the microenvironment through specific proteins produced during viral replication to supplement the oncolytic effects of the virus. Engineered HSV rQT3 has deletions of ICP6 and γ134.5 and expresses human tissue inhibitor of metallo-proteinases 3 (27). Matrix metalloproteinases are a group of endopeptidases that degrade the extracellular matrix and thus play a critical role in the tumor niche by permitting angiogenesis and invasion. RQT3-treated neuroblastoma and peripheral nerve sheath tumor xenografts not only showed delayed tumor growth but also had a reduced vascular density. Moreover, the treatment decreased circulating endothelial progenitors, indicating a possible antiangiogenic effect of the virus. Zhu et al. developed an oncolytic HSV, VAE, which carries an exogenous endo–angio fusion gene. Endostatin and angiostatin are potent angiogenesis inhibitors. The virus not only infected and killed glioma CSCs but also inhibited their vascular niche in vitro (28). Using a similar approach, Dmitrieva et al. examined the effect of Chase-ABC, an HSV that produces chondroitinase ABC, a bacterial enzyme that removes the chondroitin sulfate from proteoglycans, a major component of the tumor extracellular matrix (29). Compared with a control virus, Chase-ABCs spread throughout glioma spheroids more efficiently, and, through degradation of the extracellular matrix, the virus showed enhanced replication and antitumor activity in vivo. It is important to note that the studies using VAE and Chase-ABCs were conducted in glioma cells from adult tumors, which in general are molecularly quite distinct from pediatric gliomas, and therefore the findings might not extrapolate to pediatric glioma CSCs.
Although HSV is a neurotropic virus, it is capable of killing cells from a wide variety of nonneural cancers, including sarcomas, melanomas, colon, breast, lung, prostate, and hepatic tumors, and several adult human studies have demonstrated safety and antitumor effects (30,31,32). Preclinical efficacy of G207 in pediatric rhabdomyosarcoma (RMS) and osteosarcoma cell lines was demonstrated by Bharatan et al. (33). We have found that the CSCs, marked by CD133, in both alveolar and embryonal RMS cell lines are equally sensitive compared with other tumor cells to killing with M002 (Friedman & Gillespie, unpublished results). The humanized IL-12 version of the M002 virus, M032, is being prepared for a phase I trial at the University of Alabama at Birmingham in adult patients with recurrent GBM. No other studies to date have examined the effect of HSV on nonneural pediatric solid tumor CSCs. The first trial using an engineered HSV, HSV1716, to be injected intratumorally in pediatric patients (seven and older) with recurrent or refractory extracranial solid tumors is ongoing at Children’s Hospital Medical Center in Cincinnati (ClinicalTrials.gov identifier NCT00931931).
Adenovirus
Adenovirus is a nonenveloped, nonintegrated double-stranded DNA virus in the Adenoviridae family that has been studied extensively as a novel, oncolytic therapeutic. Whereas wild-type adenoviruses can infect both dividing and nondividing cells and cause respiratory, ophthalmic, or gastrointestinal illnesses in humans, attenuated conditionally replicative adenoviruses (CRAds) can target cancer cells with few side effects. Deletions in immediate-early (E1A) or early (E1B) adenovirus genes result in attenuated mutants that cannot bind normal cellular proteins that drive gene expression initiating and maintaining cellular proliferation needed for productive virus infection (34). These virus genome deletions do not affect viral replication in cancer cells due to pathway defects such as p16/retinoblastoma (Rb) or p53. Most adenoviral gene therapy vectors, including the most commonly used serotype 5 (Ad5), enter cells through the coxsackie adenovirus receptor (CAR), which is problematic because of highly variable (to absent) expression of CAR by tumor cells (35). For example, neuroblastoma and medulloblastomas tend to express a higher degree of CAR than gliomas, which tend to have lower and variable expression (36). Moreover, normal epithelial cells, neurons, and astrocytes also express a high amount of CAR, which could result in adverse treatment effects. Newer CRAds circumvent this limitation through modifications of the fiber knob of the viral capsid, thereby altering the tropism of the virus and enabling infection of cancer cells through a CAR-independent mechanism (34). Similar to engineered HSV-1, foreign DNA can be inserted into CRAds to enhance viral efficacy by targeting cancer cells under control of a tumor-specific promoter, by inducing a tumor-specific immune response through various cytokines, and/or by directing attacks at the tumor microenvironment and angiogenesis (35).
In preclinical and clinical studies, CRAds have demonstrated safety and efficacy in pediatric extracranial solid tumors. Ewing’s sarcoma cells expressed CAR and were highly sensitive to viral oncolysis by adenovirus (37). OBP-301 (Telomelysin), a CRAd with a human telomerase reverse transcriptase promoter driving expression of E1A and E1B genes linked to an internal ribosome entry site, was cytotoxic in osteosarcoma cell lines that expressed CAR and suppressed tumor growth in a murine osteosarcoma xenograft model (38). Telomerase plays an important role in tumorigenesis; telomerase activation results in cellular proliferation and can lead to mutagenesis and transformation, and telomerase appears to be overexpressed in CSCs compared with other tumor cells (39). Thus, therapeutics that target telomerase such as OBP-301 are promising agents to eradicate resistant CSCs.
Although there are no specific studies examining the effect of CRAds on neuroblastoma CSCs, various adenoviruses have effectively targeted neuroblastoma cells, and two viruses have been used clinically in children with neuroblastoma (40,41,42). Pesonen et al. reported treatment with an oncolytic adenovirus AD5/3-Cox2L-D24 in a 6-y-old boy with metastatic neuroblastoma resistant to several chemotherapy regimens including autologous transplant (41). The virus has a 24-base-pair deletion in the Rb binding site of E1A and the native E1A promoter is replaced with the cyclooxygenase-2 (COX-2) promoter. Cyclooxygenase-2 is believed to play an important role in tumorigenesis and cell survival by stimulating cell growth, invasiveness, and neovascularization, which are similar functions attributed to CSCs (43). Injection of 1011 viral particles into the primary tumor bed resulted in a 71% regression of the primary tumor and clearance of metastatic bone marrow disease. Side effects included mild fever, diarrhea, stomach pains, and elevated liver enzymes that resolved in 2 wk. In a separate study, four children (2–5 y of age) with refractory metastatic neuroblastoma received several doses of Ad-DM-E2F-K-Delta24RGD (ICOVIR-5), a CRAd that contains a deletion in E1A, a substitution of the E1A promoter for E2F-responsive elements, and an RGD-4C peptide motif inserted into the adenoviral fiber to enhance adenoviral tropism (42). The virus was delivered intravenously by autologous mesenchymal stem cells, which may engraft in tumor stroma, and was well tolerated with the side effect of fever in three patients and an elevated liver alanine aminotransferase in one patient that resolved in 96 h. Although three patients had no response, one patient had a very good partial response, suggesting that further investigation would be worthwhile. Currently, there are no known ongoing studies of CRAds in children.
Although not specific to pediatric brain CSCs, adenovirus vectors have shown promise in killing brain CSCs from adult glioma cell lines. Using Delta-24-RGD, a CRAd with the Rb-binding region deleted from the E1A gene and an inserted RGD (arginine-glycine-aspartic acid) into the H1 loop of the fiber protein allowing the virus to enter cells via αvβ3 and αvβ5 integrins independent of CAR, Jiang et al. demonstrated that xenografts derived from glioma CSCs were sensitive to killing by the virus, and treatment resulted in prolonged survival in glioma-bearing mice (44). The glioma CSCs expressed high levels of CAR and integrins and were targeted because of a defective Rb pathway not present in normal brain cells. Delta-24-RGD is currently in a phase I trial in adults with recurrent malignant gliomas (NCT00805376). To improve the selectivity of adenovirus for malignant gliomas, Nandi et al. developed CRAd-Survivin-pk7, an Ad5 virus with a human survivin promoter to drive E1 expression and a polylysine modification in the fiber knob to selectively bind heparan sulfate proteoglycans overexpressed in gliomas (45). Survivin is a member of the inhibitor of apoptosis family of proteins that is overexpressed on adult gliomas but downregulated in normal tissue (46). CRAd-Survivin-pk7 effectively targeted CD133+ glioma CSCs (45). In addition, the survivin promoter was radio-inducible with low-dose radiation increasing the cytotoxicity of CRAad-Survivin-pk7 in glioma cells. This effect was more pronounced in the CD133+ glioma cells, suggesting that the CSCs may have increased proliferative capacity following low-dose radiation. Of note, survivin expression in pediatric neural tumors is quite variable. Zhang et al. found that only 1 of 26 pediatric brain tumors (5 GBM, 4 low-grade astrocytomas, 10 juvenile pilocytic astrocytomas, and 7 ependymomas) showed moderate levels of survivin; however, in other studies, ependymomas, medulloblastomas, and neuroblastomas demonstrated elevated survivin expression that correlated with poor outcomes (47,48,49,50). Thus, the potential benefit of CRAd-Survivin-pk7 to target pediatric CSCs is unclear.
Skog et al. suggest that Ad5 may not be the best vector for targeting glioma CSCs, and other vectors with an adenovirus serotype 16 (Ad16) and chimpanzee serotype 23 (CV23) backbone should be evaluated as alternatives (51). Ad5 was the least efficient serotype, whereas Ad16 and CV23 were the most effective at killing both CD133+ and CD133− cells. With significant biological differences in pediatric vs. adult brain tumors, further studies are needed to determine whether pediatric brain CSCs can be effectively targeted by CRAds.
Reovirus
Reovirus (respiratory enteric orphan virus) is a nonenveloped, double-stranded, segmented RNA virus that has shown potential as an oncolytic, targeted agent. The virus only causes mild respiratory or gastrointestinal symptoms, if any, in humans. It is limited by cellular activation of protein kinase R, which subsequently phosphorylates eukaryotic initiation factor 2α, resulting in inhibition of viral gene translation and an ineffective infection in normal cells (52). Activated Ras or Ras pathway effector proteins, which are commonly found in human tumors, prevents protein kinase R activation, thereby permitting viral gene translation and resulting in an effective lytic infection. Importantly, Ras activation has been shown to be an important mediator of tumorigenesis in various tumor types and may initiate tumor formation by expanding the stem cell population (53). Thus, the benign nature of the virus, its ability to target cancer cells with upregulated Ras signaling pathway, and the capability to deliver the virus systemically make it an appealing oncolytic virus.
In preclinical studies, reovirus has been effective against pediatric malignancies. Yang et al. found most medulloblastoma (MDB) cell lines and MDB primary cultures from surgical specimens were sensitive to killing by human reovirus type 3 Dearing (54). Not only was survival significantly increased in an in vivo mouse model, but spinal and leptomeningeal metastases, which are relatively common in MDB patients, were also decreased with intrathecal injections of the virus. Using the same strain of virus, Hingorani et al. demonstrated efficacy of reovirus delivered systemically to treat rhabdomyosarcoma (RMS), osteosarcoma, and Ewing’s sarcoma cell lines in the flank of athymic nude mice (55). Combining the virus with radiation or cisplatin enhanced the therapeutic effect.
The only study examining the effects of reovirus on CSCs was in breast cancer. Breast CSCs, marked by CD24-CD44+ expression and overexpression of aldehyde dehydrogenase, were as sensitive to killing by reovirus as the non-CSCs (56). Notably, there were similar levels of Ras in the CSCs and the other tumor cells, suggesting that reovirus may effectively target chemotherapy- and radiotherapy-resistant CSCs. Further studies are needed to determine whether reovirus can effectively target CSCs from pediatric tumors.
Multiple phase I and phase II studies of reovirus injected into the tumor bed or systemically have been or are currently being conducted in adult patients with central nervous system and extracranial solid tumors (9). The virus is being tested as monotherapy or in combination with chemotherapy or radiation. Objective responses and disease stabilization have been reported with few side effects ranging from flu-like symptoms to mild gastrointestinal symptoms to neutropenia. No severe dose-limiting toxicities have been reported to date. The first trial using reovirus in pediatric patients (3–21 y of age) with relapsed or refractory extracranial solid tumors is currently accruing patients (NCT01240538; Children’s Oncology Group ADVL1014).
Seneca Valley Virus
SVV is a recently discovered single-stranded, nonenveloped, nonintegrating RNA virus in the family Picornaviridae. The conditionally replication-competent virus does not cause disease in humans but has potent cytolytic activity in some cancer cell types. The mechanism by which SVV produces a productive infection in cancer cells has not been fully elucidated; however, cell-surface receptor interactions with the virus appear to be an important component, and viral replication is at least partially mediated through autophagy (57,58). SVV has been used safely without dose-limiting toxicities in a phase I clinical trial in adults with advanced solid tumors (59).
In preclinical studies, SVV has effectively killed cancer cells in a variety of pediatric solid tumors. Reddy et al. demonstrated sensitivity of Ewing’s sarcoma, medulloblastoma, neuroblastoma, and Rb cell lines to SVV when injected systemically (57). Very high doses up to 1 × 1014 were tolerated in immunocompetent mice. Wadhwa et al. found that a single tail vein injection of SVV was able to treat invasive Rb and prevent central nervous system metastatic disease in a murine model (60). Testing of SVV by the Pediatric Preclinical Testing Program confirmed a marked cytotoxic effect of the virus in some neuroblastoma cell lines (61). In addition, RMS cell lines were highly sensitive to the virus, and there was an objective response seen in at least one rhabdoid tumor, Wilms tumor, and GBM cell line. Although several Ewing’s sarcoma cell lines were sensitive to SVV in vitro, this effect was lost in vivo. Osteosarcoma and medulloblastoma cell lines were resistant to killing by the virus. Based on the promising results by Reddy et al. and the Pediatric Preclinical Testing Program, the first trial using SVV (NCT01048892; Children’s Oncology Group ADVL0911) in children 3 to 21 y old with relapsed or refractory neuroblastoma, RMS, or rare tumors with neuroendocrine features is currently accruing patients.
Recently, Yu et al. conducted the first study of the ability of SVV to kill pediatric CSCs. In a panel of 10 primary human medulloblastoma xenografts, half of the tumors were sensitive to killing with SVV (58). The CD133+ medulloblastoma CSCs and CD133− tumor cells were equally sensitive in permissive xenografts and similarly resistant in prohibitive xenografts in all cell lines tested, suggesting that the CSCs were no more resistant to the virus than other tumor cells. The variable sensitivity of cells of the same tumor type seen by Yu et al. and the Pediatric Preclinical Testing Program should provide excellent models for determining barriers to tumor-selective replication.
Vaccinia Virus
VV is a double-stranded, enveloped DNA virus in the poxvirus family that was first used as a vaccination against smallpox and more recently has been attenuated for use as a cancer therapeutic. Mutated viruses have a deletion in both copies of the thymidine kinase (TK) gene (62). The TK-deleted virus requires thymidine triphosphate for DNA synthesis, which is provided by dividing cells, thus leading to preferential replication in dividing cells and tumor cell specificity. Another promising approach to prevent infection in normal tissue involves deleting the B18R gene, which counteracts type I interferons. The B18R-deleted mutant results in interferon-mediated enhanced virus inactivation in normal cells (63). Like HSV and adenovirus, a large portion of the genome may be replaced with foreign DNA to augment oncolysis. Several viruses that target cancer-specific antigens and/or induce an immune response through expression of various cytokines have been used in human adult trials (64). VV that express antiangiogenesis proteins have been used successfully in preclinical studies to treat human adult solid tumors (65). The first pediatric trial (NCT01169584) is currently testing the safety of JX-594, a VV that expresses human granulocyte–macrophage colony-stimulating factor to induce a tumor-specific cytotoxic immune response, in children 2 y to 21 y old with refractory or recurrent solid tumors including neuroblastoma, RMS, lymphoma, Wilms tumor, and Ewing’s sarcoma.
Two recent studies highlight the potential of VV to target and kill CSCs. Lun et al. tested JX-594 against CSCs in a panel of high-grade glioma cell lines (66). Most cells from five separate cell lines grown in serum-free medium as neurospheres, free-floating clumps of cells thought to be enriched for CSCs, were killed by the virus. The self-renewal ability of the cells grown in neurospheres was inhibited by JX-594 infection. This study is limited by the lack of pediatric glioma cell lines and the lack of a specific CSC marker used to identify the CSCs. Not all cells within a neurosphere are undifferentiated CSCs, and, in fact, most may be differentiated tumor cells. Nevertheless, the decrease in the number of neurospheres formed after infection with JX-594 suggests that the virus can target and kill some glioma CSCs. Using a Western Reserve strain of VV with mutations in TK and viral growth genes that is delivered to tumor cells by an ex vivo expanded natural killer T cell population (CIK-vvDD), Contag et al. demonstrated that CIK-vvDD targeted and killed residual murine lymphoma cells with stem-like features including the ability to initiate tumors and resistance to chemotherapy and radiation (67). No specific CSC marker exists for murine lymphoma, and no human lymphomas were used in the experiments. Therefore, further studies are necessary to determine whether this dual biotherapy can indeed target and kill human CSCs.
Newcastle Disease Virus
NDV is a negative-sense, single-stranded RNA paramyxovirus that is highly infectious in poultry but causes only mild flu-like symptoms in humans. Tumor cell tropism of the virus is thought to be dependent on defective interferon responsiveness or cell resistance to apoptosis (68,69). In preclinical studies, NDV strain 73-T selectively targeted and killed pediatric cancers including neuroblastoma, osteosarcoma, and Wilms tumor (70,71). With reverse genetic technology, recombinant viruses that express foreign genes like granulocyte–macrophage colony-stimulating factor, interferon-γ, IL-2, or tumor necrosis factor α are being tested (69).
Although there are no specific studies examining the effect of NDV on pediatric CSCs, attenuated NDV has been used safely with demonstrated efficacy in children with high-grade gliomas (72,73,74). Csatary et al. reported on three children (1.5 to 12 y old) with high-grade gliomas who received NDV MTH-68/H after failure with conventional therapies. All three patients exhibited significant tumor regression and improvement in neurological function while receiving the virus repeatedly over several years (72). These exciting results strongly suggest that further study of NDV to target pediatric malignancies would be worthwhile.
Myxoma Virus
Like VV, MYXV is a large, double-stranded DNA poxvirus that can accommodate therapeutic foreign genes by replacing up to 25 kb. The natural host range of MYXV is rabbits, and the virus does not cause disease in human but is cytotoxic to cancer cells through altered Akt signaling (75). Akt signaling plays a critical role in cell survival, growth, and proliferation, and recently the Akt pathway has been implicated in regulating the survival of CSCs following radiation (76). Furthermore, Akt inhibition has been shown to preferentially kill brain CSCs relative to other brain tumor cells and reduce tumor invasiveness (77). These data suggest that MYXV may be an excellent candidate to eradicate CSCs.
To date, there are very few studies examining the sensitivity of pediatric tumors or CSCs to MYXV. A high percentage of rhabdoid tumors, an aggressive pediatric malignancy, responded completely to a single intratumoral injection of MYXV in mice (78). Preliminary studies in neuroblastoma suggest that CSCs may be sensitive to infection by MYXV (79). Last, adult human acute myeloid leukemic stem and progenitor cells were sensitive to killing by MYXV, whereas normal hematopoietic stem and progenitor cells were not affected by the virus (80). Based on these promising studies, further evaluation of MYXV in pediatric cancers and CSCs is warranted.
Vesicular Stomatitis Virus
VSV is an enveloped, negative-sense, single-stranded RNA rhabdovirus that mainly infects livestock and only rarely causes a flu-like syndrome in humans. Similar to NDV, normal human cells are believed to be protected by the exquisite sensitivity of VSV to the host cell interferon response, whereas cancer cells may be targeted by the virus because of a loss of interferon responsiveness (81). A mutant attenuated form of the virus, VSVΔm51, which has a single amino acid deletion of methionine-51 of the matrix protein to provide additional protection for normal cells by restoring interferon-mediated responses, has shown efficacy in treating human rhabdoid tumors and gliomas (78,82). Human osteosarcoma and Ewing’s sarcoma were sensitive to infection with a different mutant VSV whereas a human synovial sarcoma line was very resistant (83). There are no reported studies on the ability of VSV to target and kill pediatric CSCs, although preliminary results suggest that neuroblastoma CSCs may be resistant (79). Further studies are needed to confirm this finding, elucidate the mechanism of resistance, and determine whether other pediatric CSCs display resistance to VSV. In addition, a recent report by Yasmeen et al. suggests that VSV may replicate well in some normal human cells; therefore, a greater understanding of VSV replication is needed before the virus is advanced to human clinical studies (84).
Future Directions
Over the past decade, great progress has been made in the field of oncolytic virotherapy. Several viruses have been translated from the laboratory to the clinic to hopefully benefit children with chemo- and radioresistant malignancies, and several more viruses are likely to be used in clinical trials in the future. Each virus has unique benefits and limitations as an oncolytic agent, which may help to determine how it is used and what tumors are targeted clinically ( Table 1 ). Beside the viruses covered in this review, several viruses, which have not been tested for efficacy against CSCs nor been used clinically in pediatric patients, including neuroattenuated poliovirus, modified measles virus, and parvovirus, have shown promise preclincally in targeting pediatric malignancies including neuroblastoma and medulloblastoma (85,86,87). With the discovery of CSCs, future research must focus on ways in which oncolytic viruses can be harnessed to target and eradicate these cells. As CSCs biology is revealed, next-generation viruses can be developed that target specific CSC antigens, the signaling pathways that regulate CSCs, and the CSC microenvironment. Table 4 summarizes potential mechanisms oncolytic viruses may use to infect and kill CSCs. Importantly, because CSCs may share many of the characteristics of nontransformed stem cells that play a vital role in developing children as well as in tissue repair and maintenance, the specificity of targeted viruses toward transformed CSCs (but not normal stem cells) must be considered as next-generation viruses are developed and oncolytic virotherapy is moved to clinical trials in children.
Strategies to enhance viral efficacy include improving virus delivery, tumor specificity, and virus replication; reducing virus clearance; and increasing the tumor-directed immune response. Combination therapy with chemotherapeutics, radiation, monoclonal antibodies, small-molecule inhibitors, and/or other oncolytic viruses will likely be necessary to eliminate CSCs and achieve superior outcomes. Low-dose chemotherapy with agents like cyclophosphamide can reduce the antiviral immune response and thus enhance oncolysis (88). Virotherapy may complement high-dose chemotherapy regimens by providing a unique cell-cycle independent mechanism of cell killing. Oncolytic viruses and radiation may act synergistically; viruses can sensitize cells to radiation, and radiation can enhance viral infection, replication, and gene expression, resulting in greater tumor cell death (89). Monoclonal antibodies and small-molecule inhibitors can complement oncolytic virotherapy by altering regulatory pathways, increasing viral replication, and enhancing the induction of apoptosis (90). Last, combination therapy with viruses that have different mechanisms of attack will likely provide a synergistic effect. Through these various combination strategies, oncolytic virotherapy offers great promise in targeting resistant CSCs and thereby decreasing toxicity and enhancing the quality of life and survival rates for children with cancer.
Statement of Financial Support
G.K.F., K.A.C., and E.A.B. are supported by the Kaul Pediatric Research Institute and the National Institutes of Health (CA071933 and CA097247).
References
Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute. (http://seer.cancer.gov/csr/1975_2005/,2008.)
Friedman GK, Gillespie GY . Cancer Stem Cells and Pediatric Solid Tumors. Cancers (Basel) 2011;3:298–318.
Sneddon JB, Werb Z . Location, location, location: the cancer stem cell niche. Cell Stem Cell 2007;1:607–11.
Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H . Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008;26:1818–30.
Moore SW . Developmental genes and cancer in children. Pediatr Blood Cancer 2009;52:755–60.
Vlashi E, McBride WH, Pajonk F . Radiation responses of cancer stem cells. J Cell Biochem 2009;108:339–42.
Rich JN, Bao S . Chemotherapy and cancer stem cells. Cell Stem Cell 2007;1:353–5.
Yang ZJ, Wechsler-Reya RJ . Hit ‘em where they live: targeting the cancer stem cell niche. Cancer Cell 2007;11:3–5.
Hammill AM, Conner J, Cripe TP . Oncolytic virotherapy reaches adolescence. Pediatr Blood Cancer 2010;55:1253–63.
Lacroix J, Witt O, Sclehofer JR, Rommelaere J . Therapeutic exploitation of oncolytic viruses in pediatric oncology. Drugs Fut 2010;35:1015.
Friedman GK, Pressey JG, Reddy AT, Markert JM, Gillespie GY . Herpes simplex virus oncolytic therapy for pediatric malignancies. Mol Ther 2009;17:1125–35.
Chou J, Kern ER, Whitley RJ, Roizman B . Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990;250:1262–6.
Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821–8.
Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 2003;100:15178–83.
Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 2005;8:323–35.
Chiou SH, Kao CL, Chen YW, et al. Identification of CD133-positive radioresistant cells in atypical teratoid/rhabdoid tumor. PLoS ONE 2008;3:e2090.
Mahller YY, Williams JP, Baird WH, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS ONE 2009;4:e4235.
Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000;7:867–74.
Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther 2000;7:859–66.
Hunter WD, Martuza RL, Feigenbaum F, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol 1999;73:6319–26.
Radbill AE, Reddy AT, Markert JM, et al. Effects of G207, a conditionally replication-competent oncolytic herpes simplex virus, on the developing mammalian brain. J Neurovirol 2007;13:118–29.
Markert JM, Coen DM, Malick A, Mineta T, Martuza RL . Expanded spectrum of viral therapy in the treatment of nervous system tumors. J Neurosurg 1992;77:590–4.
Friedman GK, Langford CP, Coleman JM, et al. Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J Neurooncol 2009;95:199–209.
Lasner TM, Kesari S, Brown SM, Lee VM, Fraser NW, Trojanowski JQ . Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J Neuropathol Exp Neurol 1996;55:1259–69.
Parikh NS, Currier MA, Mahller YY, et al. Oncolytic herpes simplex virus mutants are more efficacious than wild-type adenovirus Type 5 for the treatment of high-risk neuroblastomas in preclinical models. Pediatr Blood Cancer 2005;44:469–78.
Kambara H, Okano H, Chiocca EA, Saeki Y . An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res 2005;65:2832–9.
Mahller YY, Vaikunth SS, Ripberger MC, et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res 2008;68:1170–9.
Zhu G, Su W, Jin G, et al. Glioma stem cells targeted by oncolytic virus carrying endostatin-angiostatin fusion gene and the expression of its exogenous gene in vitro. Brain Res 2011;1390:59–69.
Dmitrieva N, Yu L, Viapiano M, et al. Chondroitinase ABC I-mediated enhancement of oncolytic virus spread and antitumor efficacy. Clin Cancer Res 2011;17:1362–72.
Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737–47.
Fong Y, Kim T, Bhargava A, et al. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol Ther 2009;17:389–94.
Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res 2010;16:4005–15.
Bharatan NS, Currier MA, Cripe TP . Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediatr Hematol Oncol 2002;24:447–53.
Ribacka C, Hemminki A . Virotherapy as an approach against cancer stem cells. Curr Gene Ther 2008;8:88–96.
Short JJ, Curiel DT . Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther 2009;8:2096–102.
Persson A, Fan X, Salford LG, Widegren B, Englund E . Neuroblastomas and medulloblastomas exhibit more Coxsackie adenovirus receptor expression than gliomas and other brain tumors. Neuropathology 2007;27:233–6.
Rice AM, Currier MA, Adams LC, et al. Ewing sarcoma family of tumors express adenovirus receptors and are susceptible to adenovirus-mediated oncolysis. J Pediatr Hematol Oncol 2002;24:527–33.
Sasaki T, Tazawa H, Hasei J, et al. Preclinical evaluation of telomerase-specific oncolytic virotherapy for human bone and soft tissue sarcomas. Clin Cancer Res 2011;17:1828–38.
Xu Y, He K, Goldkorn A . Telomerase targeted therapy in cancer and cancer stem cells. Clin Adv Hematol Oncol 2011;9:442–55.
Geoerger B, van Beusechem VW, Opolon P, et al. Expression of p53, or targeting towards EGFR, enhances the oncolytic potency of conditionally replicative adenovirus against neuroblastoma. J Gene Med 2005;7:584–94.
Pesonen S, Helin H, Nokisalmi P, et al. Oncolytic adenovirus treatment of a patient with refractory neuroblastoma. Acta Oncol 2010;49:117–9.
García-Castro J, Alemany R, Cascalló M, et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther 2010;17:476–83.
Rizzo MT . Cyclooxygenase-2 in oncogenesis. Clin Chim Acta 2011;412:671–87.
Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst 2007;99:1410–4.
Nandi S, Ulasov IV, Tyler MA, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res 2008;68:5778–84.
Van Houdt WJ, Haviv YS, Lu B, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg 2006;104:583–92.
Zhang JG, Kruse CA, Driggers L, et al. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J Neurooncol 2008;88:65–76.
Preusser M, Wolfsberger S, Czech T, Slavc I, Budka H, Hainfellner JA . Survivin expression in intracranial ependymomas and its correlation with tumor cell proliferation and patient outcome. Am J Clin Pathol 2005;124:543–9.
Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC . Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet 1998;351:882–3.
Fangusaro JR, Jiang Y, Holloway MP, et al. Survivin, Survivin-2B, and Survivin-deItaEx3 expression in medulloblastoma: biologic markers of tumour morphology and clinical outcome. Br J Cancer 2005;92:359–65.
Skog J, Edlund K, Bergenheim AT, Wadell G . Adenoviruses 16 and CV23 efficiently transduce human low-passage brain tumor and cancer stem cells. Mol Ther 2007;15:2140–5.
Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW . The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. Embo J 1998;17:3351–62.
Quinlan MP, Quatela SE, Philips MR, Settleman J . Activated Kras, but not Hras or Nras, may initiate tumors of endodermal origin via stem cell expansion. Mol Cell Biol 2008;28:2659–74.
Yang WQ, Senger D, Muzik H, et al. Reovirus prolongs survival and reduces the frequency of spinal and leptomeningeal metastases from medulloblastoma. Cancer Res 2003;63:3162–72.
Hingorani P, Zhang W, Lin J, Liu L, Guha C, Kolb EA . Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts. Cancer 2011;117:1764–74.
Marcato P, Dean CA, Giacomantonio CA, Lee PW . Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther 2009;17:972–9.
Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst 2007;99:1623–33.
Yu L, Baxter PA, Zhao X, et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro-oncology 2011;13:14–27.
Rudin CM, Poirier JT, Senzer NN, et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res 2011;17:888–95.
Wadhwa L, Hurwitz MY, Chévez-Barrios P, Hurwitz RL . Treatment of invasive retinoblastoma in a murine model using an oncolytic picornavirus. Cancer Res 2007;67:10653–6.
Morton CL, Houghton PJ, Kolb EA, et al. Initial testing of the replication competent Seneca Valley virus (NTX-010) by the pediatric preclinical testing program. Pediatr Blood Cancer 2010;55:295–303.
McCart JA, Ward JM, Lee J, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 2001;61:8751–7.
Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH . Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med 2007;4:e353.
Guse K, Cerullo V, Hemminki A . Oncolytic vaccinia virus for the treatment of cancer. Expert Opin Biol Ther 2011;11:595–608.
Wojton J, Kaur B . Impact of tumor microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev 2010;21:127–34.
Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther 2010;18:1927–36.
Contag CH, Sikorski R, Negrin RS, et al. Definition of an enhanced immune cell therapy in mice that can target stem-like lymphoma cells. Cancer Res 2010;70:9837–45.
Mansour M, Palese P, Zamarin D . Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol 2011;85:6015–23.
Ravindra PV, Tiwari AK, Sharma B, Chauhan RS . Newcastle disease virus as an oncolytic agent. Indian J Med Res 2009;130:507–13.
Reichard KW, Lorence RM, Cascino CJ, et al. Newcastle disease virus selectively kills human tumor cells. J Surg Res 1992;52:448–53.
Lorence RM, Reichard KW, Katubig BB, et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst 1994;86:1228–33.
Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol 2004;67:83–93.
Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther 2006;13:221–8.
Wagner S, Csatary CM, Gosztonyi G, et al. Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. APMIS 2006;114:731–43.
Wang G, Barrett JW, Stanford M, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA 2006;103:4640–5.
Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC . PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev 2008;22:436–48.
Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN . Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 2008;26:3027–36.
Wu Y, Lun X, Zhou H, et al. Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors. Clin Cancer Res 2008;14:1218–27.
Cripe TP, Wang PY, Marcato P, Mahller YY, Lee PW . Targeting cancer-initiating cells with oncolytic viruses. Mol Ther 2009;17:1677–82.
Kim M, Madlambayan GJ, Rahman MM, et al. Myxoma virus targets primary human leukemic stem and progenitor cells while sparing normal hematopoietic stem and progenitor cells. Leukemia 2009;23:2313–7.
Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000;6:821–5.
Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst 2006;98:1546–57.
Paglino JC, van den Pol AN . Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: rare resistance is overcome by blocking interferon pathways. J Virol 2011;85:9346–58.
Yasmeen A, Zhang L, Al Moustafa AE . Does the vesicular stomatitis virus really have a selective oncolytic effect in human cancer? Int J Cancer 2010;126:2509–10.
Toyoda H, Wimmer E, Cello J . Oncolytic poliovirus therapy and immunization with poliovirus-infected cell lysate induces potent antitumor immunity against neuroblastoma in vivo. Int J Oncol 2011;38:81–7.
Studebaker AW, Kreofsky CR, Pierson CR, Russell SJ, Galanis E, Raffel C . Treatment of medulloblastoma with a modified measles virus. Neuro-oncology 2010;12:1034–42.
Lacroix J, Leuchs B, Li J, et al. Parvovirus H1 selectively induces cytotoxic effects on human neuroblastoma cells. Int J Cancer 2010;127:1230–9.
Kumar S, Gao L, Yeagy B, Reid T . Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther 2008;10:371–9.
Touchefeu Y, Vassaux G, Harrington KJ . Oncolytic viruses in radiation oncology. Radiother Oncol 2011;99:262–70.
Nguyen TL, Tumilasci VF, Singhroy D, Arguello M, Hiscott J . The emergence of combinatorial strategies in the development of RNA oncolytic virus therapies. Cell Microbiol 2009;11:889–97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedman, G., Cassady, K., Beierle, E. et al. Targeting pediatric cancer stem cells with oncolytic virotherapy. Pediatr Res 71, 500–510 (2012). https://doi.org/10.1038/pr.2011.58
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2011.58
This article is cited by
-
Tumor Microenvironment: A Niche for Cancer Stem Cell Immunotherapy
Stem Cell Reviews and Reports (2024)
-
The role of oncolytic virotherapy and viral oncogenes in the cancer stem cells: a review of virus in cancer stem cells
Cancer Cell International (2023)
-
Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas
Cell Death & Disease (2020)
-
N-Myc expression enhances the oncolytic effects of vesicular stomatitis virus in human neuroblastoma cells
Molecular Therapy - Oncolytics (2016)
-
Cancer stem cell markers in pediatric sarcomas: Sox2 is associated with tumorigenicity in immunodeficient mice
Tumor Biology (2016)