Abstract
Preterm birth carries a risk for impaired developmental outcome. We have previously described an association between increased levels of proinflammatory cytokines during the first 72 postnatal hours and cerebral damage as detected by ultrasound in a cohort of 74 very preterm infants. Sixty-seven of 71 surviving children with a mean gestational age of 27.1 (2.0) wk were examined at 2 y corrected age with a standardized neurologic examination and with Bayley Scales of Infant Development. We hypothesized that proinflammatory cytokine concentrations at or shortly after birth would be associated with an adverse developmental outcome. Increased concentrations of TNF-α in cord blood odds ratio (95% confidence interval) 3.3 (1.1–10.2), p = 0.013 and at 6 h 7.8 (0.9–71.8), p = 0.015 and of IL-6 in cord blood 1.7 (1.0–2.9), p = 0.048 were associated with psychomotor developmental index <85. Increased concentrations of TNF-α in cord blood odds ratio (95% confidence interval) 3.6 (1.002–12.8), p = 0.044 and of IL-8 in cord blood 3.5 (1.2–10.6), p = 0.023 were associated with cerebral palsy. Associations of TNF-α and IL-8 in cord blood with the respective outcome measures remained significant after adjustment for other clinical variables. Proinflammation at birth is associated with impaired functional outcome at 2 y of corrected age in children with very preterm birth.
Similar content being viewed by others
Main
Although both survival and developmental outcome in preterm infants have improved during the last years, very preterm birth remains a major risk factor for cerebral injury and for later neurodevelopmental disability (1,2). Recent studies using MRI techniques have shown that very preterm infants have delayed maturation and volume reduction of both cerebral white and gray matter (3). Furthermore, such changes are associated with impaired neuro-developmental outcome (4–6). Intrauterine infection is a common antecedent to preterm birth and has been related to adverse neonatal outcome (7–9). In a recent study, the combination of histologic inflammation and placental vascular changes predicted abnormal neurologic outcome at 2 y of age (10).
Increased levels of proinflammatory cytokines in cord blood or in neonatal blood indicating a systemic fetal inflammatory response have been associated with development of white matter brain damage (WMD) and with CP (11,12). We have previously shown that increased levels of proinflammatory cytokines at birth and during the first 72 postnatal hours were associated with arterial hypotension, severe intraventricular hemorrhage (IVH), and WMD in very preterm infants (13). Changes in levels of cytokines occur rapidly within short time intervals (14). In the present cohort of very preterm infants, we observed that circulatory levels of cytokines were generally highest at or shortly after birth and decreased characteristically up until 72 h postnatal age. This suggested that the observed inflammatory response was initiated in utero (13).
We hypothesized that circulatory proinflammation is related to impaired developmental long-term outcome in very preterm infants and that this relationship is primarily present at or shortly after birth. Thus, we conducted a follow-up study to evaluate the relationship between proinflammatory and modulatory cytokines at birth and during the first 72 postnatal hours and neuro-developmental outcome at 2 y of age corrected for prematurity.
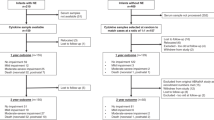
METHODS
Study population.
The study population consisted of a cohort of very preterm infants born at Lund University Hospital between February 2001 and February 2003. Seventy-four infants out of a total of 169 admitted were enrolled before delivery to participate in a study evaluating cytokine patterns during the first 72 postnatal hours (13). Inclusion criteria were GA less than 32 wk, informed parental consent, and absence of major congenital anomalies. All pregnancies were dated by ultrasound at 17–18 gestational weeks. All surviving infants from this cohort (n = 71) were asked for participation in a follow-up study at 2 y of corrected age. Sixty-seven children (94%) with a mean (SD) GA at birth of 27.1 (2.0) weeks were assessed at a corrected age of 24.0 (0.53) range (22.5–25) months. The parents of three infants declined participation and one infant was lost to follow-up. None of these infants had cerebral hemorrhage or WMD as detected by neonatal ultrasound. The study was approved by the Regional Committee for Research Ethics.
Quantitative analysis of plasma cytokines.
Blood sampling was performed from cord blood and from arterial blood at 6, 24, and 72 h postnatal age through an indwelling arterial line. Levels of proinflammatory (TNF-α, IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-12) and modulatory (IL-4, IL-10) cytokines in plasma were determined by cytometric bead array (Becton Dickinson, San Jose, CA) and flow cytometry. A level of ≤0.1 pg/mL was regarded as nondetectable. The method has been described in detail previously (13).
Cerebral ultrasound.
Repeated ultrasound examinations of the brain were performed at 1, 3, and 7 postnatal days, at 6 wk and at term age (13). Data from the ultrasound examinations were stored digitally and reviewed by a pediatric radiologist. Images with suspected abnormalities where reassessed by a second pediatric radiologist blinded to the clinical history. Cerebral hemorrhage was classified as subependymal hemorrhage (grade I) or IVH (grade II–III). WMD was defined in the presence of periventricular echodensities persisting for more than 7 d or periventricular cysts. Severe brain damage was defined as IVH grade III and/or WMD. No infants had parenchymal hemorrhage.
Antenatal and neonatal clinical data.
Antenatal and neonatal clinical data were prospectively recorded until home discharge. Premature rupture of membranes (PROM) was defined as rupture of membranes before the onset of labor and suspect maternal infection as elevated maternal C-reactive protein (>5 mg/L) and/or fever >38°C. Clinical chorioamnionitis was defined when two of the following criteria were present: maternal fever >38°C, maternal tachycardia, fetal tachycardia, malodorous amniotic fluid and uterine tenderness. The total number of doses of antenatal steroids, preeclampsia, mode of delivery, and multiple pregnancies were registered.
Gender, GA in days, birth weight for GA, Apgar score, treatment with dopamine during the first 72 h, medical treatment or surgical ligation of persistent ductus arteriosus, postnatal septicaemia, ventilator treatment (days), supplemental oxygen at 36 gestational wks and accumulated dose (mg/kg) of hydrocortisone and/or betamethasone administered from birth until discharge were registered. Retinopathy of prematurity was defined according to the international classification (15).
Data at follow-up.
CP was defined using the definitions adopted for European classification of CP (16). Children presenting previously not recognized symptoms of CP at follow-up were re-evaluated by a pediatric neurologist. When all children had reached a corrected age of 3 y the regional CP registry was interrogated to identify any additional children being diagnosed after 2 y corrected age. Maternal and paternal educational levels were defined in three categories: completed compulsory school, upper secondary education or university degree.
Neurologic and developmental outcome.
Developmental outcome was assessed by a psychologist (AH) using the Bayley Scales of Infant Development (BSID-II) with the two different index scales, Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI). A subnormal development was defined by an index score of 1 SD below the normative mean (MDI or PDI <85) and a developmental delay was defined as 2 SD below the normative mean (MDI or PDI <70). Infants with MDI or PDI scores of 50 or below were assigned a score of 50 according to the manual (17).
Neurologic assessment was performed by a neonatalist (I.H.-P.), using the Hammersmith Infant Neurologic examination, which is a method previously optimized for assessment of infants and children between 2 and 18 mo of age (18). The method is based on 26 items where each item is scored separately. The item scores can be added, thus achieving a Neurologic Optimality Score (NOS) with a maximum score of 78. A score below the 10th percentile was defined as suboptimal (NOS <74). The developmental and neurologic assessments were performed on the same day and the examiners were blinded to each other's results.
Statistical data analysis.
Statistical analysis was performed using SPSS v 15.0 for Microsoft Windows (SPSS Inc., Chicago, IL). Levels of cytokines were logarithmically transformed. Correlations between continuous variables were assessed with the Pearson correlation coefficient. Univariate analyses between clinical variables or cytokines and categorical outcome variables were assessed using χ2 test, Fischers exact test, Mann-Whitney U-test, or one sample t test as appropriate. Logistic regression was used to obtain odds ratios (95% CI) for each of the comparisons. Cytokines exhibiting significant univariate relationships with outcome variables at specific time-points were reevaluated using ANOVA for repeated measures. Cytokines and clinical variables exhibiting significant univariate associations with outcome measures were entered into multivariate analysis using logistic regression analysis (stepwise backward and forward procedures, log-likelihood ratio) to assess the best predictive model for each outcome measure.
RESULTS
Outcome measures at 2 y of age.
The median (range) scores of NOS, MDI and PDI at assessment were 71 (24–78), 86 (50–118) and 96 (50–117), respectively. The NOS correlated significantly with both the MDI and PDI, r = 0.61 (p < 0.001) and r = 0.72 (p < 0.001) respectively.
Altogether 47 children (70%) had a suboptimal NOS <74. Thirty-one children (46%) had a MDI <85 and eight children (12%) had a MDI <70. In twenty children (30%) the PDI was <85 and in seven children (10%) it was <70. All 20 children with PDI <85 also had a NOS <74.
Five children (7%) had developed CP. Two children had spastic bilateral CP (diplegia), two had spastic unilateral CP (hemiplegia), and one had dystonic CP.
Ante- and neonatal variables, parental education, and outcome at 2 y of age.
Univariate analysis identified antenatal and neonatal clinical variables associated with suboptimal NOS (<74), subnormal MDI or PDI (<85) and CP respectively (Table 1). A suboptimal NOS was associated with maternal infection, lower GA at birth and any cerebral hemorrhage. A subnormal MDI was associated with Apgar score <7 at 5 min, persistent ductus arterious, retinopathy of prematurity and increased number of ventilator days respectively whereas an increase in MABP (0–72 h), PROM and either a maternal or a paternal educational level corresponding to university degree were associated with a decreased risk for a subnormal MDI. A subnormal PDI was associated with maternal infection, lower GA at birth, Apgar score <7 at 5 min, any cerebral hemorrhage, severe brain damage and increased number of ventilator days. CP was associated with maternal infection, Apgar score <7 at 5 min, any cerebral hemorrhage and severe brain damage. No significant associations were observed between the variables preeclampsia, clinical chorioamnionitis, birth weight for GA, gender, postnatal septicemia, supplemental oxygen at 36 gestational wks and accumulated dose of hydrocortisone and/or betamethasone and any of the respective outcome measures.
Levels of cytokines at birth and outcome at 2 y of age.
Significant associations between concentrations of cytokines and outcome measures were only present in cord blood and in blood samples taken at 6 h and were restricted to concentrations of TNF-α, IL-6 and IL-8 and to the outcome measures PDI <85 and CP. Increased concentrations of TNF-α in cord blood OR (95% CI) 3.3 (1.1–10.2), p = 0.013 and at 6 h 7.8 (0.9–71.8), p = 0.015 and of IL-6 in cord blood 1.7 (1.0–2.9), p = 0.048 were associated with PDI <85. Increased concentrations of TNF-α in cord blood OR (95% CI) 3.6 (1.002–12.8), p = 0.044 and of IL-8 in cord blood 3.5 (1.2–10.6), p = 0.023 were associated with CP. These univariate relationships at specified time-points remained significant after adjustment for repeated measurements. Concentrations of TNF-α and IL-8 in cord blood and at 6, 24, and 72 h postnatal age in relation to PDI and CP are shown in Figs. 1 and 2.
Levels of TNF-α in cord blood and at 6, 24 and 72 h of postnatal age in relation to psychomotor developmental index (PDI) (A) and cerebral palsy (CP) (B) at 2 y of age. In panel A, PDI ≥85 (□: n = 47); PDI 70–84 ( : n = 13); PDI <70 (▪: n = 7). In panel B, presence of CP (▪: n = 5); no CP (□: n = 62). Medians and interquartile ranges are indicated. * = p < 0.05.
: n = 13); PDI <70 (▪: n = 7). In panel B, presence of CP (▪: n = 5); no CP (□: n = 62). Medians and interquartile ranges are indicated. * = p < 0.05.
Levels of IL-8 in cord blood and at 6, 24 and 72 h of postnatal age in relation to psychomotor developmental index (PDI) (A) and cerebral palsy (CP) (B) at 2 y of age. In panel A, PDI ≥ 85 (□: n = 47); PDI 70–84 ( : n = 13); PDI <70 (▪: n = 7). In panel B, presence of CP (▪: n = 5); no CP (□: n = 62). Medians and interquartile ranges are indicated. * = p < 0.05.
: n = 13); PDI <70 (▪: n = 7). In panel B, presence of CP (▪: n = 5); no CP (□: n = 62). Medians and interquartile ranges are indicated. * = p < 0.05.
No significant associations were observed between concentrations of IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 and any of the outcome measures.
Multivariate analysis.
Levels of cytokines were assessed together with clinical variables in relation to outcome using logistic regression analysis. A prerequisite for inclusion of a variable into multivariate analysis was a significant univariate association with the respective outcome measures. Models with the highest combined predictive capacity for the respective outcome measures are given in Tables 2–5.
A PDI <85 was best predicted by the combination of increased concentration of TNF-α in cord blood and decreasing GA. CP was best predicted by the combination of increased concentration of IL-8 in cord blood, Apgar score <7 at 5 min and severe brain damage.
DISCUSSION
The main findings of this study were that increased levels of the proinflammatory cytokines TNF-α and IL-8 in cord blood and at 6 h were associated with subnormal PDI and with CP at 2 y of corrected age in very preterm infants. These findings support that proinflammation initiated in utero is a risk factor for impaired development in preterm infants.
Serial sampling of cytokines performed at predefined time points and a high rate of participation at follow-up at 2 y of age enabled an assessment of which time-point carries the best predictive value of subsequent impairment. Concentrations of proinflammatory mediators, namely TNF-α and IL-8 in cord blood, were associated with subnormal development at 2 y. This finding may explain why previous investigators sampling inflammatory mediators at a later time point have failed to observe an association between proinflammation and subsequent neurodevelopmental impairment in preterm infants (19).
The number of infants with CP or developmental delay (MDI/PDI <70) was low thus limiting the possibility of evaluating significant associations between cytokine concentrations and severe developmental impairment at 2 y of age. The twenty children with a subnormal PDI (<85) included all who had severe IVH grade III and a large proportion of those with low Apgar score at 5 min. In addition, all infants who developed CP had a subnormal PDI and all twenty children with subnormal PDI had suboptimal NOS (<74). This infers that the applied cut-off of PDI below 85 at 2 y of age defines an outcome, which is associated with perinatal morbidity and with a risk of future developmental impairment. On the contrary, a considerably higher proportion of children (n = 47) had a suboptimal NOS (<74) which suggests that suboptimal NOS included a higher proportion of healthy infants than subnormal PDI. Nevertheless all infants with IVH grade III or CP had suboptimal NOS. Assessment of NOS was performed at a higher age than in previous study which may have affected the proportion of infants with a score defined as subnormal (18).
A limitation in the present study was the absence of placental histology. We therefore used clinical definitions such as clinical chorioamnionitis, PROM, or maternal infection as markers of antenatal inflammation. Clinical chorioamnionitis was infrequent and we did not find any significant associations with developmental outcome. Maternal infection before delivery was associated with suboptimal NOS, subnormal PDI and with CP in univariate analysis. Maternal leukocytes have the capacity to invade fetal membranes suggesting that a maternal inflammatory response may influence detected concentrations of cytokines in cord blood (20). We observed an association between maternal infection and increased levels of IL-6 in cord blood (data not shown). In an experimental model of preterm placentas, infusion of lipopolysaccharide into the maternal compartment was followed by increased TNF-α secretion on the fetal but not the maternal side (21).
In the current evaluation, PROM was protective against subnormal MDI. This is contradictory to another study of extremely preterm infants where PROM was a risk factor for low MDI whereas clinical chorioamnionitis was protective for later development of CP (22). MDI performed at 20 mo of age has poor predictive value for subsequent neurodevelopmental outcome and is also influenced by parental interaction especially in the range between 70 and 84 (23). This influence was also observed in the present study. Further, none of the variables representing cerebral damage such as severe brain damage as defined by ultrasound was associated with subnormal MDI. Cytokines were not related to MDI at any time point.
The discrepancy in associations between different antenatal risk variables associated with fetal inflammation and later developmental outcome may be explained by the timing, duration, and extent of intrauterine inflammation. The interaction between a sub-threshold insult (i.e., proinflammatory cytokines) followed by another later severe insult (i.e., hypoxia) has been described where a severe insult may result either in decreased brain injury (tolerance) or in aggravated brain injury (sensitization). The interval between the sub-threshold insult and the severe insult determines whether tolerance or sensitization occurs (24–26).
TNF-α was the cytokine most consistently showing associations with outcome measures. TNF-α plays a central role in experimental settings of brain injury and is involved in mechanisms inducing both sensitization and tolerance after lipopolysaccharide stimulation (25). TNF-α promotes brain injury through several pathways such as inhibition of migration and proliferation of neuronal precursor cells, prevention of differentiation of oligodendrocyte progenitors, induction of apoptosis and stimulation of reactive astrogliosis, all common findings in brains with white matter injury (27–29).
In a recent study, preterm infants with early WMD as detected by ultrasound and confirmed by MRI had higher cord blood concentrations of TNF-α and IL-1β (30). Both white and gray matter abnormalities detected by MRI in preterm infants have been associated with abnormal functional outcome (5). TNF-α modulates synaptic connectivity and facilitates glutamate dependent neuron death in vitro suggesting that increased concentrations of this cytokine may have a damaging effect on gray matter structures (31,32).
We have previously described an association between increased circulatory levels of the cytokine IFN-γ during the first 72 postnatal hours and WMD as detected by ultrasound in preterm infants (13). This is in line with reported increased levels of IFN-γ in cerebrospinal fluid of preterm infants with posthemorragic hydrocephalus and cystic WMD (33). Of note, no significant associations between IFN-γ and any of the outcome measures at 2 y was found although three of seven children with WMD developed CP, all with cystic periventricular leukomalacia. WMD as defined by ultrasound was neither associated with MDI (<85) nor with PDI (<85) and diffuse WMD did not relate to CP (data not shown). The weak relationship between WMD and outcome was not unexpectedly accompanied by a lack of association between IFN-γ and outcome measures. Cerebral ultrasound has been described as less reliable than MRI in defining diffuse WMD and continued evaluation of associations between components of inflammation like IFN-γ and WMD must rely on the use of MRI (34).
Induced inflammation within a short time interval before or at the time of ischemia has a sensitizing effect on the brain (24). We observed that both TNF-α in cord blood and low Apgar score at birth were univariately associated with subnormal PDI and with CP. Aggravated brain injury after intracerebral administration of TNF-α at the time of an ischemic insult has recently been shown in adult mice (25). Interestingly, we did not observe any association between decreased Apgar score and cytokine levels in cord blood (data not shown). This suggests that the two phenomena have separate etiologies but a synergistic aggravating effect on outcome. One may speculate that the combination of fetal proinflammation with increased levels of TNF-α in combination with decreased Apgar score may result in neuronal damage and thus gray matter impairment.
The associations between TNF-α and developmental outcome measures were observed although cord blood concentrations of TNF-α were generally low and frequently undetectable. We suspect that TNF-α was released and peaked in many individuals already in utero, higher levels thereby escaping detection in cord or postnatal blood (13). The adenosine system has been shown to selectively inhibit toll-like receptor mediated TNF-α production in newborn infants and to enhance the production of the anti-inflammatory cytokine IL-10 (35,36). Metabolically stressful events such as birth increase extracellular levels of adenosine (35) and we have previously shown a characteristic increase in IL-10 peaking at 6 h after preterm birth in most infants (13). Thus, the process of birth may initiate a mechanism, which obscures the detection of increased levels of TNF-α.
Increased concentration of the chemokine IL-8 in cord blood was together with low Apgar score and cerebral damage the most predictive combination of variables for development of CP whereas TNF-α was only related to CP in univariate analysis. An association between increase in IL-8 in cord blood and CP in preterm infants has been described previously (37). Further, IL-6 in cord blood did show an association with PDI <85 but this association disappeared after adjustment for severe IVH/WMD. In line with this is our previous finding of an association between increased levels of IL-6 and IL-8 and arterial hypotension as well as development of severe IVH (13). In contrast to WMD, IVH grade III was associated with both subnormal PDI and CP (data not shown) with a majority developing CP and was thus the morphologic variable most predictive of functional impairment. Increased levels of TNF-α in cord blood or at 6 h were neither associated with development of IVH nor with WMD. Correspondingly, multivariate analyses showed that the association of TNF-α with outcome measures was not established via IVH or through WMD.
Levels of IL-8 and TNF-α in infants with impaired development (i.e., PDI <85 or CP) were highest in cord blood samples. Although, presence or location of antenatal inflammation was not confirmed by placental histology or amniotic fluid sampling, the results suggest that prenatal initiation of inflammation is of importance for later impairment. In comparison to TNF-α, there is scarce support for the direct contribution of IL-8 in development of brain damage. IL-8 is relatively easy to detect and peaks later than TNF-α. After the initiation of the inflammatory stimulus, IL-8 still shows a steady increase after 48 h in vitro (14). However, in this study, significant relationships between IL-8 and outcome measures were restricted to concentrations in cord blood. Decline in levels of IL-8 after birth was varied with some infants exhibiting a very short half-life for IL-8 (data not shown). Half-life in vivo after preterm birth would thus appear considerably shorter than that described in vitro.
In conclusion, these findings support the theories of TNF-α being a key cytokine for cerebral damage in combination with hypoxic events and that proinflammation at birth in very preterm infants has implications for functional outcome. Future noninvasive methods of diagnosing fetal inflammation in utero might be of help to predict an optimal time point for delivery and open the possibility of targeting anti-inflammatory therapies with the purpose to reduce brain injury in preterm infants.
Abbreviations
- IVH:
-
intraventricular hemorrhage
- MDI:
-
mental developmental index
- NOS:
-
neurological optimality score
- PDI:
-
psychomotor developmental index
- PROM:
-
premature rupture of membranes
- WMD:
-
white matter damage
References
Wilson-Costello D, Friedman H, Minich N, Siner B, Taylor G, Schluchter M, Hack M 2007 Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000–2002. Pediatrics 119: 37–45
Marlow N, Wolke D, Bracewell MA, Samara M 2005 Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 352: 9–19
Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ 2005 Abnormal cerebral structure is present at term in premature infants. Pediatrics 115: 286–294
Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T 2003 Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res 54: 672–679
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE 2006 Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355: 685–694
Yung A, Poon G, Qiu DQ, Chu J, Lam B, Leung C, Goh W, Khong PL 2007 White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr Res 61: 732–736
Dammann O, Drescher J, Veelken N 2003 Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev Med Child Neurol 45: 148–151
Wu YW, Colford JM Jr 2000 Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 284: 1417–1424
Versland LB, Sommerfelt K, Elgen I 2006 Maternal signs of chorioamnionitis: Persistent cognitive impairment in low-birthweight children. Acta Paediatr 95: 231–235
Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, Hallman M 2006 Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res 59: 478–483
Duggan PJ, Maalouf EF, Watts TL, Sullivan MH, Counsell SJ, Allsop J, Al-Nakib L, Rutherford MA, Battin M, Roberts I, Edwards AD 2001 Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet 358: 1699–1700
Minagawa K, Tsuji Y, Ueda H, Koyama K, Tanizawa K, Okamura H, Hashimoto-Tamaoki T 2002 Possible correlation between high levels of IL-18 in the cord blood of pre-term infants and neonatal development of periventricular leukomalacia and cerebral palsy. Cytokine 17: 164–170
Hansen-Pupp I, Harling S, Berg AC, Cilio C, Hellstrom-Westas L, Ley D 2005 Circulating interferon-gamma and white matter brain damage in preterm infants. Pediatr Res 58: 946–952
Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P 2003 Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine 21: 200–206
1984 An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol 102: 1130–1134
Surveillance of Cerebral Palsy in Europe 2000 Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol 42: 816–824
Bayley N 1993 Bayley scales of Infant Development. 2nd ed. Harcourt Assessment, The Psychological corporation San Antonio, TX
Frisone MF, Mercuri E, Laroche S, Foglia C, Maalouf EF, Haataja L, Cowan F, Dubowitz L 2002 Prognostic value of the neurologic optimality score at 9 and 18 months in preterm infants born before 31 weeks' gestation. J Pediatr 140: 57–60
Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, Nelson PG, Dickens BF, Phillips TM 2003 Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res 53: 600–607
Steel JH, O'Donoghue K, Kennea NL, Sullivan MH, Edwards AD 2005 Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 26: 672–677
Holcberg G, Amash A, Sapir O, Sheiner E, Levy S, Huleihel M 2007 Perfusion with lipopolysaccharide differently affects the secretion of tumor necrosis factor-alpha and interleukin-6 by term and preterm human placenta. J Reprod Immunol 74: 15–23
Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR 2005 The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed 90: F134–F140
Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, Klein N, Friedman H, Mercuri-Minich N, Morrow M 2005 Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics 116: 333–341
Eklind S, Mallard C, Arvidsson P, Hagberg H 2005 Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res 58: 112–116
Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, Meller R, Simon RP, Stenzel-Poore MP 2007 Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab 27: 1663–1674
Wang X, Rousset CI, Hagberg H, Mallard C 2006 Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med 11: 343–353
Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N 2003 Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci 24: 623–631
Downen M, Amaral TD, Hua LL, Zhao ML, Lee SC 1999 Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia 28: 114–127
Feldhaus B, Dietzel ID, Heumann R, Berger R 2004 Effects of interferon-gamma and tumor necrosis factor-alpha on survival and differentiation of oligodendrocyte progenitors. J Soc Gynecol Investig 11: 89–96
Tsukimori K, Komatsu H, Yoshimura T, Hikino S, Hara T, Wake N, Nakano H 2007 Increased inflammatory markers are associated with early periventricular leukomalacia. Dev Med Child Neurol 49: 587–590
Jara JH, Singh BB, Floden AM, Combs CK 2007 Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J Neurochem 100: 1407–1420
Stellwagen D, Malenka RC 2006 Synaptic scaling mediated by glial TNF-alpha. Nature 440: 1054–1059
Schmitz T, Heep A, Groenendaal F, Huseman D, Kie S, Bartmann P, Obladen M, Felderhoff-Muser U 2007 Interleukin-1beta, interleukin-18, and interferon-gamma expression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus–markers of white matter damage?. Pediatr Res 61: 722–726
Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ 2003 White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol 24: 805–809
Hasko G, Cronstein BN 2004 Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25: 33–39
Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR 2006 The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol 177: 1956–1966
Huang HC, Wang CL, Huang LT, Chuang H, Liu CA, Hsu TY, Ou CY, Yang KD 2004 Association of cord blood cytokines with prematurity and cerebral palsy. Early Hum Dev 77: 29–36
Acknowledgements
We thank Frances Cowan for valuable advice regarding the neurologic examination, Jeanette Arvastsson for technical help with CBA and flow cytometry and Per-Erik Isberg for help with statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Swedish Medical Research Council (grant nrs 14940, 4732), Lund University Hospital and Lund Medical Faculty grants, the Gorthon Foundation, the Linnéa and Josef Carlsson foundation, and the Nils W. Svenningsen Foundation for Preterm Infants.
Rights and permissions
About this article
Cite this article
Hansen-Pupp, I., Hallin, AL., Hellström-Westas, L. et al. Inflammation at Birth is Associated With Subnormal Development in Very Preterm Infants. Pediatr Res 64, 183–188 (2008). https://doi.org/10.1203/PDR.0b013e318176144d
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318176144d
This article is cited by
-
CpG methylation patterns in placenta and neonatal blood are differentially associated with neonatal inflammation
Pediatric Research (2023)
-
Chorioamnionitis and neonatal outcomes
Pediatric Research (2022)
-
Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice
Scientific Reports (2018)
-
The role of systemic inflammation linking maternal BMI to neurodevelopment in children
Pediatric Research (2016)
-
TNF-α and MTHFR Polymorphisms Associated with Cerebral Palsy in Chinese Infants
Molecular Neurobiology (2016)