Abstract
This study was aimed at describing abdominal and laryngeal muscle responses to upper airway occlusion (UAO) in early life and the effect of sleep states on these responses. Twelve nonsedated, 9-26-d-old lambs were studied. We simultaneously recorded 1) airflow (pneumotachograph + face mask);2) sleep states (electrocorticogram and electrooculogram);3) abdominal muscle (external obliquus) electromyogram (EMG); and 4) glottic constrictor (thyroarytenoid) and dilator (posterior cricoarytenoid and cricothyroid) muscle EMGs. The pneumotachograph was repeatedly occluded for 15-30 s in wakefulness and natural sleep. We analyzed 90 occlusions during wakefulness (11 lambs), 28 during non-rapid eye movement(nREM) sleep (six lambs), and 23 during rapid eye movement (REM) sleep (five lambs). A phasic expiratory external obliquus EMG was present during baseline and progressively increased throughout UAO in wakefulness and nREM sleep, but not in REM sleep. Phasic thyroarytenoid EMG progressively increased during inspiratory efforts throughout UAO in wakefulness and nREM sleep, paralleling the increase in glottic dilator (posterior cricoarytenoid and cricothyroid) EMG. In contrast, glottic muscle response to UAO in REM sleep was severely blunted or disorganized by frequent swallowing movements. We conclude that UAO triggers complex and coordinated laryngeal and abdominal muscle responses during wakefulness and nREM sleep in lambs; these responses are largely absent, however, in REM sleep. These unique results, together with the defective arousal response in REM sleep, suggest that vulnerability to airway occlusion could be increased during REM sleep in early life. Possible implications for understanding severe postnatal apneas are discussed.
Similar content being viewed by others
Main
Current hypotheses favor a leading role for UAO in apnea of prematurity(1), apparent life-threatening events(2), and SIDS(3–6). Thus, a significant decrease in SIDS cases after recommendations to avoid prone position for nursing infants is interpretated as a strong argument for implicating UAO by bedclothing in SIDS(7). Establishment of oral breathing in cases of nasal obstruction, and arousal from sleep when obstruction cannot be relieved while sleeping, are probably crucial for survival at this early age(8).
Other respiratory responses against UAO are also seemingly important in early life. Inspiratory efforts involving thoracic muscles (diaphragm and intercostal muscles) and upper airway dilating muscles (genioglossus, PCA) have been reported(9). Interestingly, these responses are depressed in REM sleep(8,10), which is the prominent sleep stage in early life. However, laryngeal muscle behavior and expiratory efforts in response to UAO have not been thoroughly studied, especially as a function of sleep state in early life.
The aim of this study was to further describe Abd and laryngeal muscle responses to external UAO in nonsedated lambs and to determine the effects of sleep on these responses. Results indicate that UAO triggers 1) expiratory efforts involving Abd activity and 2) complex and coordinated response from various laryngeal muscles. These responses are disorganized or absent in REM sleep.
METHODS
Animals. We studied 12 mixed-breed lambs after natural full-term delivery, aged 9-26 d (mean ± SD = 14 ± 5) with a mean weight of 6.4 ± 2 kg. Each lamb was housed in our animal quarters with its mother up to the day of the experiment. All procedures were approved by our institution's Ethics Committee for Animal Experimentation.
Surgical preparation. Surgery was performed 2-4 d before experimentation under general anesthesia (Fluothan 2%-N2O 30%). Atropin sulfate (0.2 mg/kg s.c.) was systematically given preoperatively and repeated every 30 min during the procedure.
Bipolar enameled chrome wire EMG electrodes (0.1-mm diameter, Chromel, GTSM, Castelnaudary, France) were inserted into intrinsic laryngeal and abdominal muscles as previously reported(11,12). Briefly, an EMG electrode was sewn under direct vision into both TAs through a small window made on each side of the thyroid cartilage (12 lambs). Other electrodes were inserted in the PCA (seven lambs) after rotating the larynx, through an incision made in the inferior pharyngeal constrictor, and in the CT(five lambs). Two other EMG electrodes were inserted into right Abd (external obliquus) (six lambs). The leads were s.c. tunneled to exit on the back of the animals.
Three stainless steel screws for ECoG recording were screwed bilaterally in the frontal and parietal bones, and their tips were positioned at the contact of the dura mater. Portions of the screw extruding from the skull were embedded in glue (Rapid Adhesive X 60, Hottingen Badwin Messtechnik, Darmstadt, Germany). Two screws were used for ECoG recording, whereas the remaining screw served as a ground. Finally, under local anesthesia two platinum subdermal needle electrodes (F-E2, Grass Instrument Company, Quincy, MA) were placed into the superior and inferior eyelids just before the start of the experiment for eye movement recording.
Measurement apparatus. A face mask, specifically molded for each lamb, was attached to a size 0 pneumotachograph (Hewlett-Packard 21070B, Palo Alto, CA) to record airflow (˙V). Tidal volume(VT) was calculated by an electronic integrator(Hewlett-Packard 8815A respiratory integrator, Palo Alto, CA). Raw EMG signals were amplified and 30- to 1000-Hz band pass filtered (Grass P511 AC preamplifier and 7 DADC drive amplifier, Quincy, MA) before undergoing a 100-ms moving time averaging (Department of Electronics, Faculty of Medicine, Université de Sherbrooke).
Tidal volume (VT), airflow (˙V), raw and integrated EMGs, ECoG, and eye movement signals were recorded on a 10-channel polygraph (Grass 7D, Quincy, MA). In addition, ˙V and integrated EMG signals were parallely fed into an IBM compatible microcomputer(Televideo-Telecat-286, Sunnyvale, CA), where they were digitized (sampling rate, 40 Hz) and analyzed. The collected data were stored on a disk for further analysis.
Experimental design. The study allowed us to infer glottic and Abd response to upper airway occlusion in nonsedated lambs during wakefulness and nREM and REM sleep.
Experiments were performed during evening and night in a silent environment. Lambs were placed in a sling in the prone position. After adjustment of the face mask, the lamb's head was carefully secured in a comfortable and naturally adopted posture, taking care not to apply pressure on the hypopharyngeal and laryngeal regions. UAOs were performed randomly during wakefulness, nREM sleep, and REM sleep by digital occlusion of the pneumotachograph. When possible, multiple occlusions for 15, 20, 25, and 30 s were performed in each sleep state. Occlusions were prematurely stopped if arousal with gross agitation occurred. Lambs were allowed at least 2 min to regain baseline breathing between two occlusions. Careful observation of lambs was conducted throughout the experiment for behavioral state staging (see below). Occlusions were performed at least 2 min after the lamb entered nREM sleep and 30 s after entering REM sleep.
Data analysis. Throughout the test, airflow (˙V, mL·kg-1·min-1), tidal volume(VT, mL·kg-1), breathing frequency(f, min-1), and duty cycle of inspiratory time/total time(Ti/Ttot) were computed for each breath and averaged for 15-s epoch. Each value was expressed as mean ± SD.
Arousal occurrence before the end of occlusion was noted, and the effect of sleep state on arousal occurrence was analyzed using a χ2 test(Statview IV, Abacus Concepts, Berkeley, CA).
The integrated EMG phasic activity of Abd and glottic muscles was carefully measured and averaged on three successive respiratory efforts just before occlusion (baseline value), during occlusion (at beginning, middle, and end of occlusion), at the moment of breathing resumption, and 10 s after ending the occlusion. Average values for each above defined EMG were then calculated for each sleep stage and expressed as percentage of the maximal recorded amplitude in each lamb.
Swallowing was identified by the typical TA burst in association with interruption of airflow and/or mouth pressure and/or other respiratory muscle EMG. For each occlusion, number of swallows were calculated during the 10 s preceding occlusion, during occlusion (expressed as number per 10 s), and during 10 s after occlusion. The effect of state of consciousness on swallow frequency was compared using analysis of variance, completed by a post hoc test (Student-Newman-Keuls) or contrast comparison as required(SuperANOVA, Abacus Concepts, Berkeley, CA).
Usual electrophysiologic and behavioral criteria were used to define state of consciousness; wakefulness was recognized by the presence of a high frequency, low voltage ECoG pattern with open eyes and occasional movements. A slow frequency, high voltage ECoG pattern with closed eyes and absence of movements characterized nREM sleep. REM sleep was recognized by the presence of a high frequency, low voltage ECoG pattern, with rapid eye movements and occasional body twitches. Arousal from nREM sleep was characterized by sudden disappearance of high amplitude waves in the ECoG; arousal from REM sleep was indicated by sudden reappearance of tonic activity on CT, PCA, and/or Abd EMGs. Arousal was always confirmed by direct observation of the lamb. A p value < 0.05 was considered significant for statistical analyses.
RESULTS
A total of 243 UAOs were performed during 101 periods of wakefulness, 111 periods of nREM sleep, and 41 periods of REM sleep in 12 lambs. Arousal prematurely terminated occlusion significantly more often during nREM sleep than during REM sleep (76 versus 35% of occlusions, respectively, p< 0.001). Moreover, there was a trend for arousal after a shorter delay in nREM sleep than in REM sleep (9.37 s ± 6.1 versus 13.25 s ± 4, respectively). After rejecting occlusions with agitation (body movements, cough, and chewing) or arousal from sleep because of inability to perform muscle analysis, the respiratory muscle response was analyzed during 90 occlusions in wakefulness (11 lambs), 28 occlusions in nREM sleep (six lambs), and 23 occlusions in REM sleep (five lambs).
Wakefulness. Baseline room air breathing. Average values for ventilatory parameters in 11 lambs during room air breathing were f = 42.6 ± 6.1 min-1, VT = 13.2 ± 2.6 mL·kg-1, VE = 543± 78 mL·kg-1min-1 and Ti/Ttot = 0.43 ± 0.03.
Phasic Abd EMG was consistently present in all lambs (n = 6) during expiration throughout baseline recording, although EMG amplitude varied with activity of the animal. Careful observation of TA EMG showed no phasic respiratory TA activity (7/11 lambs) or low amplitude phasic inspiratory activity (4/11 lambs). Phasic inspiratory glottic dilator EMG (PCA,n = 7, and CT, n = 5) and tonic EMG during expiration were consistently observed during each breath in all lambs studied.
Effect of UAO. Each external UAO (15-30-s duration) was associated with a decrease in breathing frequency and increase in Ti/Ttot throughout occlusion. Mask pressure recordings in three lambs showed progressively increasing inspiratory and expiratory efforts from the beginning to the end of occlusion, with highest values observed during the last occluded effort (Fig. 1). Although the first respiratory effort was always inspiratory, it was slightly delayed when occlusion was performed during or at end inspiration. During wakefulness, mask pressure measured during the last occluded inspiratory effort was higher in occlusions of longer duration. The average decrease in transcutaneous Hb saturation in O2(two lambs) was 14% (range 8-18%) after UAO of 25-s duration.
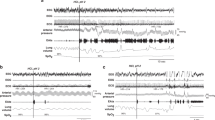
TA and Abd response to UAO in one lamb during wakefulness. TA, raw TA EMG; ∫TA, integrated TA EMG;˙V, airflow (inspiration upward); Pm, mask pressure; ABD, raw Abd EMG; ∫ABD, integrated Abd EMG; *=swallowing movement. Note the progressively increasing mask pressure and Abd muscle EMG throughout occlusion.
In all lambs, UAO consistently induced Abd phasic EMG with a progressive increase from the first occluded expiratory effort to the last. After breathing resumption, Abd expiratory EMG decreased rapidly to regain baseline values in less than 1 min (Fig. 1).
Phasic inspiratory TA EMG progressively increased in 83 out of 90 UAO (Figs. 2 and 3). When absent during unoccluded breathing, inspiratory TA EMG usually appeared within the second or third occluded inspiratory effort; on occasion, it was observed near the end of occlusion, although less frequently. TA inspiratory EMG amplitude during the last occluded efforts was higher with longer occlusions. In four lambs, there was no TA phasic EMG, either during baseline room air breathing or during occlusions (n = 7). Both PCA (Fig. 4) and CT (Fig. 3) phasic inspiratory EMGs consistently and progressively increased during occlusion, beginning with the first occluded effort; furthermore, tonic expiratory PCA and CT EMGs were increased throughout occlusion.
Progressive increase in TA phasic EMG during upper airway occlusion in wakefulness. Inspiration is upward. See legend of Figure 1 for abbreviations.
Simultaneous increase in CT and TA EMG with inspiratory diaphragmatic efforts against occlusion in an awake lamb. See legend of Figure 1 for abbreviations. Di, raw diaphragm EMG; ∫Di, integrated Di EMG; VT, tidal volume (inspiration upward); CT, raw CT EMG, ∫CT, integrated CT EMG. EEG, electroencephalogram; EOG, electrooculogram.
Representative tracing of glottic and abdominal muscle EMGs during UAO in wakefulness. Inspiration is upward. See legend of Figures 1 and 3 for abbreviations; PCA, raw PCA EMG; ∫PCA, integrated PCA EMG. Note that while UAO elicited an increase in Abd EMG with expiratory efforts, PCA EMG increased simultaneously with TA EMG during inspiratory efforts.
Swallow frequency was not increased during occlusion, but increased after breathing resumption. The effect of adding O2 for 10 min before occlusions to prevent hypoxia from developing during UAO was tested in two lambs during wakefulness. UAOs of longer duration (up to 90 s) were tolerated without any agitation or apparent discomfort by both lambs. Preventing hypoxia did not appear to modify responses to UAO, either for breathing patterns, Abd, or laryngeal muscle EMGs (Fig. 5).
TA and Abd EMG response to UAO in an awake lamb rendered hyperoxic by breathing pure O2 for 10 min preceding occlusion. Inspiration is upward. See legend of Figure 1 for abbreviations. (A) Beginning of occlusion; (B) end of occlusion and breathing resumption (actual duration of occlusion: 85 s). Note that TA and Abd response is similar to the one observed in normoxic conditions.
nREM sleep. Baseline room air breathing. Baseline room air breathing during nREM sleep was characterized by regular respiration with f = 40 ± 6.3 min-1, VT= 12.5 ± 1.4 mL·kg-1, ˙VE= 498 ± 79 mL·kg-1·min-1 and Ti/Ttot = 0.42 ± 0.04. Regular Abd phasic expiratory EMG was present in all three studied lambs throughout unoccluded respiration.
Low amplitude TA inspiratory EMG was present in two of six lambs but absent in the four remaining lambs. Phasic inspiratory EMG of the glottic dilator muscles (PCA and CT) and tonic PCA expiratory EMG were generally similar to those recorded during wakefulness, with infrequent higher or lower amplitude. Surprisingly, tonic CT expiratory EMG was absent for some periods of unoccluded breathing in nREM. Swallow frequency before occlusion was not significantly different in nREM sleep compared with wakefulness.
Effect of UAO. Twenty-eight UAOs without arousal were analyzed in six lambs during nREM sleep, with an occlusion duration between 15 and 25 s. Respiratory responses to UAO during nREM were qualitatively similar to those observed during wakefulness, i.e. decreased breathing frequency, increased Ti/Ttot, and progressively increasing inspiratory and expiratory efforts throughout occlusion. The average decrease in transcutaneous hemoglobin saturation in O2 (two lambs) was 17% after UAO of 25-s duration. Augmented expiratory efforts during UAO were consistently accompanied by progressively increasing Abd phasic expiratory EMG, which rapidly returned to baseline values after resumption of unoccluded breathing (Fig. 6).
Time-course of the Abd and glottic muscle response(mean ± SEM) to UAO during wakefulness (□-□), nREM(⋄·····⋄), and REM sleep (○-○), expressed as percentage of maximal EMG for each muscle. See legend of Figures 1,3,and 4 for abbreviations. Abd, three lambs; TA, five lambs; PCA, three lambs; CT, three lambs. BL, baseline; BO, beginning of occlusion;MID, middle of occlusion; END, end of occlusion;BR, breathing resumption; 10s, 10 s after BR. Each point and bar represents the mean value and SEM.
TA response to UAO was similar to that observed during wakefulness in four of six lambs, with inspiratory EMG progressively increasing throughout UAO (Figs. 6 and 7). In the two remaining lambs, TA response to occlusion was very weak or completely absent. Similar to that observed during wakefulness, both PCA and CT phasic inspiratory EMGs progressively increased throughout occlusion, beginning with the first occluded effort; furthermore, tonic PCA and CT expiratory EMGs were increased (Figs. 6 and 7). Swallow frequency was not increased during or after occlusion in nREM sleep.
Rem sleep. Baseline room air breathing. REM sleep periods were characterized by irregular breathing movements frequently interrupted by swallowing and chewing movements and by body twitches. During unoccluded breathing, f = 48 ± 11 min-1,VT = 9.8 ± 0.8 mL·kg-1,˙VE = 444 ± 123 mL·kg-1.min-1 and Ti/Ttot = 0.42 ± 0.04.
Abd EMG was virtually absent during REM sleep in all three studied lambs, especially during bursts of rapid eye movements. Irregular TA phasic inspiratory and/or expiratory EMG of variable amplitude was present during REM sleep (four of five lambs). This irregular respiratory activity was frequently interrupted by high amplitude EMG during swallowing movements, in contrast to nREM sleep where swallowing activity was seldomly observed. Highly irregular PCA and CT inspiratory EMGs paralleled irregular breathing patterns; however, phasic inspiratory and tonic expiratory EMGs of both PCA and CT were usually of higher amplitude than that observed during wakefulness and nREM sleep (Fig. 6). Swallow frequency (per 10 s) before occlusion was significantly increased in REM sleep (1.65 ± 1.4) as compared to nREM sleep (0.37 ± 0.26) and wakefulness (0.1 ± 0.15)(p < 0.05).
Effect of UAO. We analyzed 23 UAOs (15-30-s duration) without arousal or change in sleep state in five lambs. In four lambs, UAO did not elicit consistent respiratory efforts, although particularly frequent swallowing movements were present (Fig. 8). In the remaining lamb, progressively increasing respiratory efforts were observed throughout occlusion. The average decrease in transcutaneous Hb saturation in O2 (two lambs) was 19% (range 16-24%) after UAO of 25-s duration. Abd phasic EMG (n = 3 lambs) was virtually absent during UAO, except in one lamb where an irregular, low amplitude Abd expiratory EMG was observed (Figs. 6 and 9).
UAO had no effect on TA EMG in three lambs, in whom frequent swallowing activity remained unchanged throughout occlusion (Fig. 8). In one lamb, the TA phasic inspiratory/expiratory EMG decreased from baseline, whereas in another lamb TA inspiratory EMG progressively increased throughout occlusion. Glottic dilator (CT, n = 3, and PCA,n = 3) response to UAO was weak or absent (Figs. 6 and 9) and frequently interrupted by swallowing, the latter not significantly altered by occlusion (Fig. 8).
DISCUSSION
Our experimental design brings unique insight into laryngeal and abdominal responses to upper airway occlusion in non sedated lambs. It appears that both groups of glottic muscles (dilator and constrictor muscles) and abdominal muscles are involved in a complex and coordinated response to upper airway occlusion in early life. Furthermore, although these respiratory responses are essentially preserved in nREM sleep, they appear to be severely blunted or absent during REM sleep.
Respiratory Responses to UAO
As previously reported by others(13), UAO during wakefulness in the lambs used in this study triggered respiratory responses, among which increased Ti/Ttot, decreased breathing frequency, and increased inspiratory efforts have already been well described. Therefore emphasis will be placed on thoracic and laryngeal muscle response to UAO.
Thoracic muscles. An inspiratory accessory muscle recruitment with UAO has been previously reported for intercostal and sternomastoid muscles in children with obstructive sleep apnea syndrome(14) and for intercostal muscles in lambs(10). It is generally agreed that the increase in thoracic inspiratory muscle activity will help to support the diaphragm and hence decrease the likelihood of diaphragm fatigue. This could be especially relevant in infants who are known to have an easily fatiguable diaphragm, due to muscular fiber type and shape of the lower rib cage (i.e. horizontal ribs)(15).
Abdominal muscle recruitment in expiration in response to UAO has been largely ignored until now, although it probably contributes significantly in the response to UAO(16, 17), and this by at least two different mechanisms. First, Abd expiratory contraction could support diaphragmatic inspiratory efforts; indeed, increased abdominal pressure during expiratory efforts would place the diaphragm on more advantageous portion of its length-tension curve for beginning the next inspiratory effort, thus helping to prevent diaphragm fatigue(18). Second, Abd expiratory contraction could raise“expiratory” pressure within the airways, thus working to reestablish or preserve upper airway patency.
Laryngeal muscles. The present data showing increased phasic PCA EMG with inspiratory efforts are in agreement with previous data on increased upper airway dilator muscle activity during inspiratory efforts, including pharyngeal (genioglossus)(13,19) and glottic (PCA)(9) dilator muscles. It is generally agreed that this helps to preserve upper airway patency, whereas forceful“inspiratory” contractions of thoracic muscles induce highly negative pressure within the airways. The CT recruitment during inspiratory efforts in our lambs is also probably aimed at maintaining upper airway patency. Indeed, although contraction of the CT alone leads to glottic constriction, simultaneous contraction of CT and PCA is synergistic to glottic dilation(20). Our CT muscle results in lambs extend previous data in anesthetized adult dogs showing inspiratory CT recruitment with inspiratory resistive loading(21).
The simultaneous increase in TA during inspiratory efforts appears more intriguing, TA being well characterized as a glottic constrictor that typically contracts during the first part of expiration(9, 22). However, phasic inspiratory TA activity has already been described by others in several experiments. Hence, phasic inspiratory electrical activity has been recorded from the nerve to the TA in decerebrate cats(23). Also, the arytenoidus muscle(another glottic constrictor in which contraction would be expected in expiration) also exhibits phasic inspiratory EMG in adult humans in eupnea during wakefulness(24). Finally, although TA has been reported to be absent during nasal obstruction in lambs(9), increased inspiratory TA EMG has been observed with inspiratory resistive loads during wakefulness(25) or during obstructive sleep apneas(26) in adult humans, in agreement with the present data.
The explanation for inspiratory TA recruitment in response to UAO is less clear. It appears that the classic distinction between glottic dilator muscles contracting in inspiration versus glottic constrictor muscles contracting in expiration is perhaps inaccurate. In agreement with Kuna et al.(22), we believe that vocal cord position at each instant most probably results from simultaneous, coordinated contraction of several glottic muscles, dilators, and constrictors. During inspiratory efforts with increased glottic dilator muscle (PCA and CT) contraction, it may be that TA contraction does not result in a narrower glottis. It is also possible that TA contraction may be synergistic to PCA and CT for glottic dilation under these specific conditions; however, this hypothesis remains to be demonstrated.
Effect of REM Sleep on Responses to UAO
Arousal response. It has been repeatedly shown that REM sleep is responsible for blunting responses to UAO in young mammals. This is especially true for delaying the arousal response, as reviewed recently by Harding et al.(8) and shown again by our results. There is general belief that arousal from obstructive apnea is probably crucial for survival in early life, when other responses do not reinitiate upper airway patency(8).
Respiratory muscle response. Although respiratory muscle responses are not greatly affected in nREM sleep compared with wakefulness, REM sleep is known to significantly blunt respiratory muscle activity, through active central inhibition of thoracic postural muscles and upper airway muscles.
Thoracic muscles. Our observation that the progressive Abd expiratory recruitment with UAO in lambs disappears in REM sleep is in agreement with previous data. Indeed, Abd expiratory recruitment in response to hypercapnia in infants(27) or to UAO in children(17) was also observed in nREM sleep, but not in REM sleep. This inhibition during REM sleep is also true for other thoracic respiratory muscles, the postural function of which is important. Hence, the inspiratory recruitment of intercostal muscles in lambs(10) and sternomastoid muscles in children(14,16) observed with UAO in nREM sleep disappeared in REM sleep. As discussed above, lack of Abd recruitment during UAO in REM sleep (and of other thoracic muscles) could promote diaphragm fatigue.
Laryngeal muscles. It has been previously shown that the inspiratory response to UAO of pharyngeal (genioglossus) and laryngeal (PCA) dilators is decreased in REM sleep(13,19). Results from present experiments extend those findings to cricothyroid and thyroarytenoid muscles. Moreover, we observed that CT and TA were frequently involved in swallowing function during REM sleep, irrespective of respiratory efforts against UAO. We hypothesize that the absence of coordinated CT and TA recruitment with respiratory efforts during UAO, together with frequent swallows, could further promote pharyngeal/laryngeal occlusion.
Swallows and upper airway occlusion. Swallow frequency has been previously reported to be increased immediately before and during obstructive apneas in preterm infants(28, 29). Although airway secretions could cause both swallowing and apnea, the underlying mechanisms responsible for enhancing that protective reflex during apnea remain unclear(28). In the present study, although swallow frequency was increased in REM sleep compared with both wakefulness and nREM sleep, airway occlusion did not increase swallows in any state. Accordingly, our results suggest that increased swallow frequency in lambs is more linked to REM sleep in itself than to airway occlusion. Increased swallow frequency during REM sleep has been previously reported in adult humans(30) and in preterm infants(28). Swallowing activity during REM sleep in lambs could be another expression of the phasic motor activity characteristic of REM sleep (as myoclonic twitches, REMs, sucking movements)(31). Alternatively, swallows could be due to increased upper airway secretions in REM. However, to our knowledge, this has never been shown previously.
Evidence accumulated so far suggests that REM sleep could have a detrimental effect for the respiratory (this study) and arousal(8) responses against UAO, especially when repetitive UAOs are present. Such conditions are numerous during the early postnatal period: apneas of prematurity(32) or infancy(4, 5), postoperative apnea in former preterm infants(33), gastroesophageal reflux(34), cervicofacial abnormality(35), respiratory occlusion during sleeping in prone position(36), more generally an apparent life-threatening event(2), and perhaps SIDS(3). In these situations, lack of thoracic inspiratory and expiratory muscle recruitment during UAO in REM sleep could promote diaphragm fatigue, whereas absent and/or disorganized upper airway muscle activity could promote further UAO. Finally, the association with a delayed arousal response in REM sleep could significantly increase the importance of inadequate respiratory muscle response to UAO.
In conclusion, our results in lambs suggest that abdominal muscles and the different laryngeal muscles are significantly involved in respiratory responses to UAO during wakefulness and nREM sleep in early life. Moreover, the likely detrimental effect of REM sleep on these responses is also observed. Our observations could have potential importance for the understanding of events leading from repetitive UAO to brain damage in premature babies and perhaps to an apparent life-threatening event and SIDS in infants.
Abbreviations
- SIDS:
-
sudden infant death syndrome
- REM:
-
rapid eye movement
- nREM:
-
non-rapid eye movement
- EMG:
-
electromyogram
- TA:
-
thyroarytenoid muscle
- PCA:
-
posterior cricoarytenoid muscle
- CT:
-
cricothyroid muscle
- Abd:
-
abdominal muscle
- ECoG:
-
electrocorticogram
- UAO:
-
upper airway occlusion
References
Ruggins NR 1991 Pathophysiology of apnea in preterm infants. Arch Dis Child 66: 70–73.
Ruggins NR, Milner AD 1993 Site of upper airway obstruction in infants following an acute life-threatening event. Pediatrics 91: 595–601.
Freed GE, Steinschneider A, Glassman M, Winn K 1994 Sudden infant death syndrome prevention and an understanding of selected clinical issues. Pediatr Clin North Am 41: 967–990.
Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A 1992 Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep 15: 287–292.
Sullivan CE, McNamara F, Waters KA, Harris M, Everett F, Seton C, Bruderer J 1993 Nasal CPAP: use in the management of infantile apnea. Sleep 16:S108–S111.
Guilleminault C, Stoohs R, Skrobal A, Labanowski M, Simmons J 1993 Upper airway resistance in infants at risk for sudden infant death syndrome. J Pediatr 122: 881–886.
Mitchell EA 1993 Sleeping position of infants and the sudden infant death syndrome. Acta Paediatr 389 ( suppl): 26–30.
Harding R, Jakubowska AE, McCrabb GJ 1995 Postnatal development of responses to airflow obstruction. Clin Exp Pharm Physiol 22: 537–543.
Harding R, Buttress JA, Caddy DJ, Glen AW 1987 Respiratory and upper airway responses to nasal obstruction in awake lambs and ewes. Respir Physiol 68: 177–188.
Henderson-Smart DJ, Read DJC 1976 Depression of respiratory muscles and defective responses to nasal obstruction during active sleep in the newborn. Aust Paediat J 12: 261–266.
Praud JP, Kianicka I, Leroux JF, Dalle D 1995 Laryngeal response to hypoxia in awake lambs during the first postnatal days. Pediatr Res 37: 482–488.
Praud JP, Canet E, Kianicka I, Gaultier CI, Bureau MA 1993 Vagal and chemoreceptor influences on abdominal muscle activity in awake lambs during hypoxia. J Appl Physiol 74: 1689–1696.
Carlo WA, Miller MJ, Martin RJ 1985 Differential response of respiratory muscles to airway occlusion in infants. J Appl Physiol 59: 847–852.
Praud JP, Monrigal JP, Delaperche MF, D'Allest AM, Nedelco H, Gaultier Cl 1988 Respiratory muscle activity in children with obstructive sleep apnea syndrome. In: Chouard C (ed) Proceedings, 1st International Congress on Rhonchopathy. J Libbey Eurotext Ltd, pp 59–63.
Bryan C, Wohl MEB 1986 Respiratory mechanics in children. In: Macklem PT, Mead J (eds) Handbook of Physiology, Section 3: The Respiratory System, Vol III, Part 2. American Physiological Society, Bethesda MD, pp 179–192.
Jeffries B, Brouillette RT, Hunt CE 1984 Electromyographic study of some accessory muscles of respiration in children with obstructive sleep apnea. Am Rev Respir Dis 129: 696–702.
Praud JP, D'Allest AM, Nedelcoux H, Curzi-Dascalova L, Guilleminault C, Gaultier Cl 1989 Sleep-related abdominal muscle behavior during partial or complete obstructed breathing in prepubertal children. Pediatr Res 26: 347–350.
De Troyer A 1983 Mechanical action of the abdominal muscles. Bull Eur Physiopathol Respir 19: 575–581.
Gauda EB, Miller MJ, Carlo WA, DiFiore JM, Martin RJ 1989 Genioglossus and diaphragm activity during obstructive apnea and airway occlusion in infants. Pediatr Res 26: 583–587.
Konrad HR, Rattenborg CC 1969 Combined action of laryngeal muscles. Acta Oto-laryngol 67: 646–649.
Woodson GE, Powell FL 1991 Effects of hypoxia and hypercapnia on cricothyroid muscle response to airway pressure. Respir Physiol 87: 25–35.
Kuna ST, Insalaco G, Woodson GE 1988 Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol 65: 1332–1339.
Zhou D, Huang Q, St John WM, Bartlett D Jr 1989 Respiratory activities of intralaryngeal branches of the recurrent laryngeal nerve. J Appl Physiol 67: 1171–1178.
Kuna ST, Insalaco G, Villeponteaux RG 1991 Arytenoidus muscle activity in normal adult humans during wakefulness and sleep. J Appl Physiol 70: 1655–1664.
Insalaco G, Kuna ST, Costanza BM, Catania G, Cibella F, Bellia V 1991 Thyroarytenoid muscle activity during loaded and nonloaded breathing in adult humans. J Appl Physiol 70: 2410–2416.
Insalaco G, Kuna ST, Catania G, Marrone O, Costanza BM, Bellia V, Bonsignore G 1993 Thyroarytenoid muscle activity in sleep apneas. J Appl Physiol 74: 704–709.
Praud JP, Egreteau L, Benlabed M, Curzi-Dascalova L, Nedelcoux H, Gaultier Cl 1991 Abdominal muscle activity during CO2 rebreathing in sleeping neonates. J Appl Physiol 70: 1344–1350.
Thach BT, Menon A 1985 Pulmonary protective mechanisms in human infants. Am Rev Respir Dis 131: S55–S58.
Pickens DL, Schefft G, Thach BT 1988 Prolonged apnea associated with upper airway protective reflexes in apnea of prematurity. Am Rev Respir Dis 137: 113–118.
Lichter I, Muir RC 1975 The pattern of swallowing during sleep. Electroencephalogr Clin Neurophysiol 38: 427–432.
Moruzzi G 1963 Active processes in the brainstem during sleep. Harvey Lect 58: 233–297.
Poets CF, Samuels MP, Southall DP 1994 Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate 65: 211–219.
Coté CJ, Zaslavsky A, Downes JJ, Kurth CD, Welborn LG, Warner LO, Malviya SV 1995 Postoperative apnea in former preterm infants after inguinal herniorrhaphy. Anesthesiology 82: 809–822.
Menon AP, Schefft GL, Thach BT 1985 Apnea associated with regurgitation in infants. J Pediatr 106: 625–629.
Roberts JL, Reed WR, Mathew OP, Thach BT 1986 Control of respiratory activity of the genioglossus muscle in micrognathic infants. J Appl Physiol 61: 1523–1533.
Waters KA, Gonzalez A, Jean C, Morielli A, Brouillette RT 1996 Face-straight-down and face-near-straight-down positions in healthy, prone-sleeping infants. J Pediatr 128: 616–625.
Acknowledgements
The authors thank Bruno Gagné for technical assistance. We are also grateful to Dr. Véronique Diaz for critical review of the manuscript.
Author information
Authors and Affiliations
Additional information
Supported by the Medical Research Council of Canada Grant 7137. I.K. and J.-P.P. are scholars of the Fonds de Recherche en Santé du Québec.
Rights and permissions
About this article
Cite this article
Kianicka, I., Praud, JP. Influence of Sleep States on Laryngeal and Abdominal Muscle Response to Upper Airway Occlusion in Lambs. Pediatr Res 41, 862–871 (1997). https://doi.org/10.1203/00006450-199706000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199706000-00011