Abstract
Background:
During the last 30 years, there has been a major shift in initial staging in prostate cancer (CaP) in Western countries, with the incidence of metastases at diagnosis decreasing from over 50% in the 1970s to currently less than 10%. Yet, CaP is still the second cause of cancer death in men. We used two monthly curated databases of patients with castration-resistant prostate cancer (CRPC) to describe the natural history of patients dying of CaP in the modern era.
Methods:
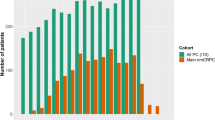
The outcome of 190 men with metastatic CRPC treated from 2008 to 2011 was studied. The characteristics of the patients who died from CaP (n=113 patients, 61%) were analyzed.
Results:
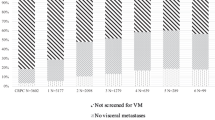
All 113 patients who died of CaP were assessable for the presence of metastases at diagnosis. Sixty-three patients (56%) had detectable metastases at diagnosis: 67%, 11% and 43% had bone, visceral and lymph node metastases, respectively. The median time to CRPC was 16 months and median overall survival (OS) was 5.2 years.
Among the patients with localized CaP at diagnosis (n=50, 44%), 46% had T stage⩾3 and 38% had a Gleason score⩾8. Overall, 64% of patients were classified as having a high-risk CaP. Only 26% who died from CaP had a Gleason score⩽6. Median OS was 8.8 years.
Conclusions:
In the modern era, approximately half of the patients who die from CaP have metastases at diagnosis. The paradigm of progression from localized disease to metastasis and eventually death is only represented in the other half, although possible initial screening and staging errors ought to be taken into consideration. More efforts are needed to conduct trials in patients with newly diagnosed metastatic CaP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel R, Naishadham N, Jemal A . Cancer Statistics, 2012. CA Cancer J Clin 2012; 62: 10–29.
Postma R, van Leenders A, Roobol MJ, Schröder FH, van der Kwast TH . Tumor features in the control and screening arm of a randomized trial of prostate cancer. Eur Urol 2006; 50: 70–75.
Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012; 366: 981–990.
Chowdhury S, Robinson D, Cahill DD, Rodriguez-Vida A, Holmberg L, Møller H . Causes of death in men with prostate cancer: an analysis of 50 000 men from the Thames Cancer Registry. BJU Int 2013; 112: 182–189.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374–1403.
Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K . What do prostate cancer patients die of? Oncologist 2011; 16: 175–181.
Epstein M, Edgren G, Rider JR, Mucci LA, Adami HO . Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst 2012; 104: 1335–1342.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280: 969–974.
Johansson JE, Andrén O, Andersson SO, Dickman PW, Holmberg L, Magnuson A et al. Natural history of early, localized prostate cancer. JAMA 2004; 291: 2713–2719.
Millikan RE, Wen S, Pagliaro LC, Brown MA, Moomey B, Do KA et al. Phase 3 trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol 2008; 26: 5936–5942.
Wang J, Halford S, Rigg A, Roylance R, Lynch M, Waxman J . Adjuvant mitoxantrone chemotherapy in advanced prostate cancer. BJU Int 2000; 86: 675–680.
Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Estemi B et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomized, open-label, phase 3 trial. Lancet Oncol 2013; 14: 149–158.
NLM Identifier NCT00268476. STAMPEDE: Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy: A Multi-Stage Multi-Arm Randomized Controlled Trial.
Sweeney C, Chen YH, Carducci MA, Jarrrad DF, Eisenberg MA, Wong YN et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. J Clin Oncol 2014; 32: 5 s (suppl; abstr LBA2).
NLM Identifier NCT01957436. A prospective randomized phase III study of androgen deprivation therapy with or without local radiotherapy with or without abiraterone acetate and prednisone in patients with metastatic hormone-naïve prostate cancer.
NLM Identifier NCT01715285. A study of abiraterone plus low-dose prednisone plus androgen-deprivation therapy (ADT) versus ADT alone in newly-diagnosed patients with high-risk, metastatic hormone-naïve prostate cancer (mHNPC).
Montgomery RB, Joshua A, Hannah AL, Peterson AC, Lopez C, Gleave ME et al. A randomized, open-label, phase II study of MDV3100 alone or in combination with leuprolide and dutasteride as neoadjuvant therapy to prostatectomy in intermediate and high-risk prostate cancer. J Clin Oncol 2012; 30 (suppl; abstr TPS4695).
Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013; 123: 4918–4922.
Kruck S, Gakis G, Stenzl A . Circulating and disseminated tumor cells in the management of advanced prostate cancer. Adv Urol 2012; 2012: 135281.
Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H et al. Perioperative activation of disseminated tumor cell in bone marrow of patients with prostate cancer. J Clin Oncol 2009; 27: 1549–1556.
Osisami M, Keller ET . Mechanisms of metastatic tumor dormancy. J Clin Med 2013; 2: 136–150.
Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7: 472–479.
Grimm MO, Kamphausen S, Hugenschmidt H, Stephan-Odenthal M, Ackermann R, Vögeli TA . Clinical outcome of patients with lymph node-postive cancer after radical prostatectomy versus androgen deprivation. Eur Urol 2002; 41: 628–634.
Gjertson CK, Asher KP, Sclar JD, Goluboff ET, Olsson CA, Benson MC et al. Local control and long-term disease-free survival for stage D1 (T2-T4N1-N2M0) prostate cancer after radical prostatectomy in the PSA Era. Urology 2007; 70: 723–727.
Fizazi K, Lesaunier F, Delva R, Gravis G, Rolland F, Priou F et al. A phase III trial of docetaxel-estramustine in high-risk localized prostate cancer: a planned analysis of response, toxicity and quality of life in the GETUG 12 trial. Eur J Cancer 2012; 18: 209–217.
NLM Identifier NCT01952223. A Phase III of Cabazitaxel and Pelvic Radiotherapy in Localized Prostate Cancer and High-risk Features of Relapse (PEACE2).
Taplin M, Montgomery RB, Logothetis C, Bubley GJ, Richie JP, Dalkin BL et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. J Clin Oncol 2012; 30 (suppl; abstr 4521).
Efstathiou E, Davis JW, Troncoso P, Titus MA, Hoang A, Wen S et al. Cytoreduction and androgen signaling modulation by abiraterone acetate (AA) plus leuprolide acetate (LHRHa) versus LHRHa in localized high-risk prostate cancer (PCa): preliminary results of a randomized preoperative study. J Clin Oncol 2012; 30 (suppl; abstr 4556).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Patrikidou, A., Loriot, Y., Eymard, JC. et al. Who dies from prostate cancer?. Prostate Cancer Prostatic Dis 17, 348–352 (2014). https://doi.org/10.1038/pcan.2014.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2014.35
This article is cited by
-
Endocrine consequences of treatment with the new androgen receptor axis-targeted agents for advanced prostate cancer
Hormones (2021)
-
The expression and functional analysis of the sialyl-T antigen in prostate cancer
Glycoconjugate Journal (2020)
-
Performance of clinicopathologic models in men with high risk localized prostate cancer: impact of a 22-gene genomic classifier
Prostate Cancer and Prostatic Diseases (2020)
-
Fracture risk and survival outcomes in metastatic castration-resistant prostate cancer patients sequentially treated with abiraterone acetate and RADIUM-223
European Journal of Nuclear Medicine and Molecular Imaging (2020)
-
Sites of synchronous distant metastases and prognosis in prostate cancer patients with bone metastases at initial diagnosis: a population‐based study of 16,643 patients
Clinical and Translational Medicine (2019)