Abstract
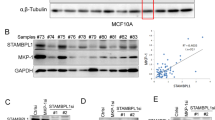
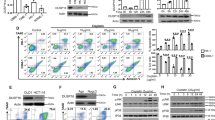
Chemoresistance is a major cause of treatment failure and poor outcome in neuroblastoma. In this study, we investigated the expression and function of dual-specificity phosphatase 26 (DUSP26), also known as mitogen-activated protein kinase phophatase-8, in human neuroblastoma. We found that DUSP26 was expressed in a majority of neuroblastoma cell lines and tissue specimens. Importantly, we found that DUSP26 promotes the resistance of human neuroblastoma to doxorubicin-induced apoptosis by acting as a p53 phosphatase to downregulate p53 tumor suppressor function in neuroblastoma cells. Inhibiting DUSP26 expression in the IMR-32 neuroblastoma cell line enhanced doxorubicin-induced p53 phosphorylation at Ser20 and Ser37, p21, Puma, Bax expression as well as apoptosis. In contrast, DUSP26 overexpression in the SK-N-SH cell line inhibited doxorubicin-induced p53 phosphorylation at Ser20 and Ser37, p21, Puma, Bax expression and apoptosis. Using in vitro and in vivo assays, we found that DUSP26 binds to p53 and dephosphorylates p53 at Ser20 and Ser37. In this report, we show that DUSP26 functions as a p53 phosphatase, which suppresses downstream p53 activity in response to genotoxic stress. This suggests that inhibition of this phosphatase may increase neuroblastoma chemosensitivity and DUSP26 is a novel therapeutic target for this aggressive pediatric malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Amano T, Nakamizo A, Mishra SK, Gumin J, Shinojima N, Sawaya R et al. (2009). Simultaneous phosphorylation of p53 at serine 15 and 20 induces apoptosis in human glioma cells by increasing expression of pro-apoptotic genes. J Neurooncol 92: 357–371.
Brodeur GM . (2003). Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3: 203–216.
Brooks CL, Gu W . (2003). Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol 15: 164–171.
Chehnab NH, Malikazy A, Stravridi ES, Halazonetis TD . (1999). Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA 96: 13777–13782.
Chen L, Malcolm AJ, Wood KM, Cole M, Variend S, Cullinane C et al. (2007). p53 is nuclear and functional in both undifferentiated and differentiated neuroblastoma. Cell Cycle 6: 2685–2696.
Eischen CM, Lozano G . (2009). p53 and MDM2: antagonists or partners in crime? Cancer Cell 15: 161–162.
Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A . (2003). Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol 162: 1807–1815.
Goldman SC, Chen CY, Lowsing TJ, Gilmer TM, Kastan MB . (1996). The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization. Am J Pathol 148: 1381–1385.
Hupp TR, Lane DP, Ball KL . (2000). Strategies for manipulating the p53 pathway in the treatment of human cancer. Biochem J 352 (Part 1): 1–17.
Jeffrey KL, Camps M, Rommel C, Mackay CR . (2007). Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov 6: 391–403.
Kruse JP, Gu W . (2009). Modes of p53 regulation. Cell 137: 609–622.
Levine AJ . (1997). p53, the cellular gatekeeper for growth and division. Cell 88: 323–331.
Li DW, Liu JP, Schmid PC, Schlosser R, Feng H, Liu WB et al. (2006). Protein serine/threonine phosphatase-1 dephosphorylates p53 at Ser-15 and Ser-37 to modulate its transcriptional and apoptotic activities. Oncogene 25: 3006–3022.
Li L, Ljungman M, Dixon JE . (2000). The human Cdc14 phosphatases interact with and dephosphorylate the tumor suppressor protein p53. J Biol Chem 275: 2410–2414.
London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H et al. (2005). Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol 23: 6459–6465.
Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE et al. (1994). p53 status and the efficacy of cancer therapy in vivo. Science 266: 807–810.
Lu X, Nannenga B, Donehower LA . (2005). PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev 19: 1162–1174.
Maruta H, Holden J, Sizeland A, D'Abaco G . (1991). The residues of Ras and Rap proteins that determine their GAP specificities. J Biol Chem 266: 11661–11668.
Matthay KK, Perez C, Seeger RC, Brodeur GM, Shimada H, Atkinson JB et al. (1998). Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. J Clin Oncol 16: 1256–1264.
Moll UM, LaQuaglia M, Benard J, Riou G . (1995). Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc Natl Acad Sci USA 92: 4407–4411.
Patterson KI, Brummer T, O'Brien PM, Daly RJ . (2009). Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 418: 475–489.
Pestell KE, Ducruet AP, Wipf P, Lazo JS . (2000). Small molecule inhibitors of dual specificity protein phosphatases. Oncogene 19: 6607–6612.
Shang X, Burlingame SM, Okcu MF, Ge N, Russell HV, Egler RA et al. (2009). Aurora A is a negative prognostic factor and a new therapeutic target in human neuroblastoma. Mol Cancer Ther 8: 2461–2469.
Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A et al. (2005). The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci USA 102: 731–736.
Toledo F, Wahl GM . (2006). Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6: 909–923.
Tweddle DA, Malcolm AJ, Bown N, Pearson AD, Lunec J . (2001). Evidence for the development of p53 mutations after cytotoxic therapy in a neuroblastoma cell line. Cancer Res 61: 8–13.
Tweddle DA, Pearson AD, Haber M, Norris MD, Xue C, Flemming C et al. (2003). The p53 pathway and its inactivation in neuroblastoma. Cancer Lett 197: 93–98.
Unger T, Juven-Gershon T, Moallem E, Berger M, Vogt Sionov R, Lozano G et al. (1999). Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J 18: 1805–1814.
Vasudevan SA, Skoko J, Wang K, Burlingame SM, Patel PN, Lazo JS et al. (2005). MKP-8, a novel MAPK phosphatase that inhibits p38 kinase. Biochem Biophys Res Commun 330: 511–518.
Vasudevan SA, Shang X, Chang S, Ge N, Diaz-Miron JL, Russel HV et al. (2009). Neuroblastoma derived secretory protein is a novel secreted factor overexpressed in neuroblastoma. Mol Cancer Ther 8: 2478–2489.
Vogelstein B, Lane D, Levine AJ . (2000). Surfing the p53 network. Nature 408: 307–310.
Vousden KH, Lu X . (2002). Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604.
Yu W, Imoto I, Inoue J, Onda M, Emi M, Inazawa J . (2007). A novel amplification target, DUSP26, promotes anaplastic thyroid cancer cell growth by inhibiting p38 MAPK activity. Oncogene 26: 1178–1187.
Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK et al. (2008). Phosphorylation of Thr-178 and Thr- 184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem 283: 24497–24505.
Acknowledgements
We thank Dr Xiangwei Wu for providing GFP-p53 expression plasmid and Mdm2-Luc reporter. The work was supported in part by the grants from the Hope Street Kids Foundation (to JY), the Bear Necessities Pediatric Cancer Foundation (to JY), the American Cancer Society Grant RSG-06-070-01-TBE (to JY), the Society of University Surgeons Ethicon Resident Scholarship (SAV) and the NIH/NCI-NRSA training grant 1F32CA113059-01A1 (SAV).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Shang, X., Vasudevan, S., Yu, Y. et al. Dual-specificity phosphatase 26 is a novel p53 phosphatase and inhibits p53 tumor suppressor functions in human neuroblastoma. Oncogene 29, 4938–4946 (2010). https://doi.org/10.1038/onc.2010.244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.244
Keywords
This article is cited by
-
Dusp26 phosphatase regulates mitochondrial respiration and oxidative stress and protects neuronal cell death
Cellular and Molecular Life Sciences (2022)
-
MYCN amplification and ATRX mutations are incompatible in neuroblastoma
Nature Communications (2020)
-
NEAP/DUSP26 suppresses receptor tyrosine kinases and regulates neuronal development in zebrafish
Scientific Reports (2017)
-
Protein serine/threonine phosphatase PPEF-1 suppresses genotoxic stress response via dephosphorylation of PDCD5
Scientific Reports (2017)
-
Role of miRNAs in the pathogenesis and susceptibility of diabetes mellitus
Journal of Human Genetics (2017)