Abstract
Many proteins contain targeting signals within their sequences that specify their delivery to particular organelles. The peroxisomal targeting signal-1 (PTS1) is a C-terminal tripeptide that is sufficient to direct proteins into peroxisomes. The PTS1 sequence closely approximates Ser-Lys-Leu-COO−. PEX5, the receptor for PTS1, interacts with the signal via a series of tetratricopeptide repeats (TPRs) within its C-terminal half. Here we report the crystal structure of a fragment of human PEX5 that includes all seven predicted TPR motifs in complex with a pentapeptide containing a PTS1 sequence. Two clusters of three TPRs almost completely surround the peptide, while a hinge region, previously identified as TPR4, forms a distinct structure that enables the two sets of TPRs to form a single binding site. This structure reveals the molecular basis for PTS1 recognition and demonstrates a novel mode of TPR–peptide interaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
van den Bosch, H., Schutgens, R.B.H., Wanders, R.J.A. & Tager, J.M. Annu. Rev. Biochem. 61, 157–197 (1992).
Gould, S.J. & Valle, D. Trends Genet. 16, 340–345 (2000).
Gould, S.J., Keller, G.A., Hosken, N., Wilkinson, J. & Subramani, S. J. Cell Biol. 108, 1657–1664 (1989).
Dodt, G. et al. Nature Genet. 9, 115–125 (1995).
Terlecky, S.R., Nuttley, W.M., McCollum, D., Sock, E. & Subramani, S. EMBO J. 14, 3627–3634 (1995).
Gatto, G.J., Jr., Geisbrecht, B.V., Gould, S.J. & Berg, J.M. Proteins 38, 241–246 (2000).
Goebl, M. & Yanagida, M. Trends Biochem. Sci. 16, 173–177 (1991).
Blatch, G.L. & Lässle, M. BioEssays 21, 932–939 (1999).
Lamb, J.R., Tugendreich, S. & Hieter, P. Trends Biochem. Sci. 20, 257–259 (1995).
Hirano, T., Kinoshita, N., Morikawa, K. & Yanagida, M. Cell 60, 319–328 (1990).
Sikorski, R.S., Boguski, M.S., Goebl, M. & Hieter, P. Cell 60, 307–317 (1990).
Das, A.K., Cohen, P.W. & Barford, D. EMBO J. 17, 1192–1199 (1998).
Scheufler, C. et al. Cell 101, 199–210 (2000).
Schliebs, W. et al. J. Biol. Chem. 274, 5666–5673 (1999).
Swinkels, B.W., Gould, S.J. & Subramani, S. FEBS Lett. 305, 133–136 (1992).
Braverman, N., Dodt, G., Gould, S.J. & Valle, D. Hum. Mol. Genet. 7, 1195–1205 (1998).
Shimozawa, N. et al. Biochem. Biophys. Res. Commun. 262, 504–508 (1999).
Blobel, G. & Dobberstein, B. J. Cell Biol. 67, 835–851 (1975).
Keenan, R.J., Freymann, D.M., Walter, P. & Stroud, R.M. Cell 94, 181–191 (1998).
Clemons, W.M., Jr., Gowda, K., Black, S.D., Zwieb, C. & Ramakrishnan, V. J. Mol. Biol. 292, 697–705 (1999).
Batey, R.T., Rambo, R.P., Lucast, L., Rha, B. & Doudna, J.A. Science 287, 1232–1239 (2000).
Conti, E., Uy, M., Leighton, L., Blobel, G. & Kuriyan, J. Cell 94, 193–204 (1998).
Opperdoes, F.R. Annu. Rev. Microbiol. 41, 127–151 (1987).
Keller, G.A. et al. J. Cell Biol. 114, 893–904 (1991).
de Walque, S., Kiel, J.A., Veenhuis, M., Opperdoes, F.R. & Michels, P.A. Mol. Biochem. Parasitol. 104, 106–119 (1999).
Geisbrecht, B.V., Zhang, D., Schulz, H. & Gould, S.J. J. Biol. Chem. 274, 21797–21803 (1999).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T.C. & Berendzen, J. Acta Crystallogr. D 55, 849–861 (1999).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 50, 760–763 (1994).
Cowtan, K. Joint CCP4 and ESF–EACBM Newsletter. Protein Crystallogr. 31, 34–38 (1994).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeeldgard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Esnouf, R.M. J. Mol. Graphics 15, 132–134 (1997).
Nicholls, A., Sharp, K.A. & Honig, B. Proteins 11, 281–296 (1991).
Acknowledgements
We thank D. Leahy, J. Beneken, and C. Dann for technical assistance and many helpful discussions, L. Brand, D. Toptygin, and A. Russo for the use of and assistance with the spectrofluorometer, N. Zachara for assistance with the SMART analysis, and A. Guerrerio for valuable assistance with the design of the anisotropy experiments and data analysis. This work was supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatto, G., Geisbrecht, B., Gould, S. et al. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat Struct Mol Biol 7, 1091–1095 (2000). https://doi.org/10.1038/81930
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81930
This article is cited by
-
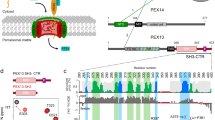
Modulation of peroxisomal import by the PEX13 SH3 domain and a proximal FxxxF binding motif
Nature Communications (2024)
-
Peroxisomal compartmentalization of amino acid biosynthesis reactions imposes an upper limit on compartment size
Nature Communications (2023)
-
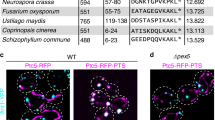
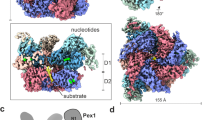
Peroxisome biogenesis initiated by protein phase separation
Nature (2023)
-
Small molecule mediated inhibition of protein cargo recognition by peroxisomal transport receptor PEX5 is toxic to Trypanosoma
Scientific Reports (2022)
-
A missense allele of PEX5 is responsible for the defective import of PTS2 cargo proteins into peroxisomes
Human Genetics (2021)