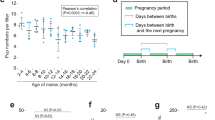
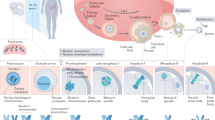
Abstract
Several studies have demonstrated a decline in the male reproductive system, sperm quality, and fertility with advancing paternal age, yet many of the biological mechanisms that underlie this process remain poorly understood. It is unclear whether the problem arises from the progenitor spermatogonial stem cells (for example, from an accumulation of DNA damage and mutations), from the somatic niche present in the testis (consisting of Sertoli and peritubular myoid cells), or from a combination of the two. Current data, albeit from a small number of studies, suggest that both factors have a role in age-associated germ cell loss. What is clear, on the other hand, is that mounting evidence links paternal age to chromosomal damage and genetic problems in the children of older fathers. The frequency of de novo mutations increases markedly with age, leading to increased risk of breast cancer, cardiac defects, developmental disorders, behavioural disorders, and neurological disease in the children of older men. The current trend towards fathering children at a later age raises concerns regarding the risk of offspring developing complex multigene diseases.
Key Points
-
Male reproductive function declines with age, as evidenced by decreased sperm quality and increased time to pregnancy
-
The quality and quantity of spermatogonial stem cells seems to decline with age, undoubtedly affecting the quality of germ cells and, ultimately, sperm
-
Alterations in DNA repair pathways in the ageing male occur concomitantly with observations of increased DNA damage in germ cells and mature sperm
-
Ageing males display increased rates of de novo mutations, which are linked to an increased risk of diseases such as autism and schizophrenia in offspring
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wright, W. W., Fiore, C. & Zirkin, B. R. The effect of aging on the seminiferous epithelium of the brown Norway rat. J. Androl. 14, 110–117 (1993).
Wang, C. et al. Reproductive aging in the Brown Norway rat is characterized by accelerated germ cell apoptosis and is not altered by luteinizing hormone replacement. J. Androl. 20, 509–518 (1999).
Molina, R. I. et al. Semen quality and aging: analysis of 9,168 samples in Cordoba, Argentina. Arch. Esp. Urol. 63, 214–222 (2010).
Cardona Maya, W., Berdugo, J. & Cadavid Jaramillo, A. The effects of male age on semen parameters: analysis of 1,364 men attending an andrology center. Aging Male 12, 100–103 (2009).
Kidd, S. A., Eskenazi, B. & Wyrobek, A. J. Effects of male age on semen quality and fertility: a review of the literature. Fertil. Steril. 75, 237–248 (2001).
Syntin, P. & Robaire, B. Sperm structural and motility changes during aging in the Brown Norway rat. J. Androl 22, 235–244 (2001).
de La Rochebrochard, E., de Mouzon, J., Thepot, F. & Thonneau, P. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil. Steril. 85, 1420–1424 (2006).
de La Rochebrochard, E., McElreavey, K. & Thonneau, P. Paternal age over 40 years: the “amber light” in the reproductive life of men? J. Androl 24, 459–465 (2003).
Ford, W. C. et al. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood). Hum. Reprod. 15, 1703–1708 (2000).
Hassan, M. A. & Killick, S. R. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil. Steril. 79, (Suppl. 3), 1520–1527 (2003).
Klonoff-Cohen, H. S. & Natarajan, L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am. J. Obstet. Gynecol. 191, 507–514 (2004).
Thacker, P. D. Biological clock ticks for men too: genetic defects linked to sperm of older fathers. JAMA 291, 1683–1685 (2004).
Sloter, E. D. et al. Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertil. Steril. 87, 1077–1086 (2007).
Hultman, C. M. et al. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry 16, 1203–1212 (2011).
Sipos, A. et al. Paternal age and schizophrenia: a population based cohort study. BMJ 329, 1070 (2004).
Clermont, Y. The cycle of the seminiferous epithelium in man. Am. J. Anat. 112, 35–51 (1963).
Johnson, L. Spermatogenesis and aging in the human. J. Androl. 7, 331–354 (1986).
Gosden, R. G., Richardson, D. W., Brown, N. & Davidson, D. W. Structure and gametogenic potential of seminiferous tubules in ageing mice. J. Reprod. Fertil. 64, 127–133 (1982).
Wang, C., Leung, A. & Sinha-Hikim, A. P. Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology 133, 2773–2781 (1993).
Ryu, B. Y., Orwig, K. E., Oatley, J. M., Avarbock, M. R. & Brinster, R. L. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24, 1505–1511 (2006).
Paniagua, R., Nistal, M., Saez, F. J. & Fraile, B. Ultrastructure of the aging human testis. J. Electron Microsc. Tech. 19, 241–260 (1991).
Chen, H., Hardy, M. P., Huhtaniemi, I. & Zirkin, B. R. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J. Androl. 15, 551–557 (1994).
Suzuki, N. & Withers, H. R. Exponential decrease during aging and random lifetime of mouse spermatogonial stem cells. Science 202, 1214–1215 (1978).
Zhang, X., Ebata, K. T., Robaire, B. & Nagano, M. C. Aging of male germ line stem cells in mice. Biol. Reprod. 74, 119–124 (2006).
Ehmcke, J., Joshi, B., Hergenrother, S. D. & Schlatt, S. Aging does not affect spermatogenic recovery after experimentally induced injury in mice. Reproduction 133, 75–83 (2007).
Boyle, M., Wong, C., Rocha, M. & Jones, D. L. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1, 470–478 (2007).
Richardson, B. Impact of aging on DNA methylation. Ageing Res. Rev. 2, 245–261 (2003).
Oakes, C. C., Smiraglia, D. J., Plass, C., Trasler, J. M. & Robaire, B. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc. Natl Acad. Sci. USA 100, 1775–1780 (2003).
Maegawa, S. et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 20, 332–340 (2010).
Rakyan, V. K. et al. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 20, 434–439 (2010).
Teschendorff, A. E. et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 20, 440–446 (2010).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Gil, J. & Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat. Rev. Mol. Cell Biol. 7, 667–677 (2006).
Quelle, D. E., Zindy, F., Ashmun, R. A. & Sherr, C. J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Matheu, A. et al. Anti-aging activity of the Ink4/Arf locus. Aging Cell 8, 152–161 (2009).
Molofsky, A. V. et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 (2006).
Baarends, W. M., van der Laan, R. & Grootegoed, J. A. DNA repair mechanisms and gametogenesis. Reproduction 121, 31–39 (2001).
Fisher, H. M. & Aitken, R. J. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J. Exp. Zool. 277, 390–400 (1997).
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362, 709–715 (1993).
Bauche, F., Fouchard, M. & Jegou, B. Antioxidant system in rat testicular cells. FEBS Lett. 349, 392–396 (1994).
Wood, R. D., Mitchell, M. & Lindahl, T. Human DNA repair genes, 2005. Mutat. Res. 577, 275–283 (2005).
Jaroudi, S. & SenGupta, S. DNA repair in mammalian embryos. Mutat. Res. 635, 53–77 (2007).
Schmid, T. E. et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum. Reprod. 22, 180–187 (2007).
Singh, N. P., Muller, C. H. & Berger, R. E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 80, 1420–1430 (2003).
Vazquez-Memije, M. E., Capin, R., Tolosa, A. & El-Hafidi, M. Analysis of age-associated changes in mitochondrial free radical generation by rat testis. Mol. Cell Biochem. 307, 23–30 (2008).
Weir, C. P. & Robaire, B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J. Androl. 28, 229–240 (2007).
de Lamirande, E. & Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl 16, 21–25 (1993).
Aitken, R. J., Harkiss, D., Knox, W., Paterson, M. & Irvine, D. S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 111 (Pt 5), 645–656 (1998).
Aitken, R. J. & Clarkson, J. S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J. Reprod. Fertil. 81, 459–469 (1987).
Aitken, R. J. et al. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 59, 1037–1046 (1998).
Gomez, E. et al. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 17, 276–287 (1996).
Zubkova, E. V. & Robaire, B. Effects of ageing on spermatozoal chromatin and its sensitivity to in vivo and in vitro oxidative challenge in the Brown Norway rat. Hum. Reprod. 21, 2901–2910 (2006).
Wyrobek, A. J. et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl Acad. Sci. USA 103, 9601–9606 (2006).
Brahem, S., Mehdi, M., Elghezal, H. & Saad, A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J. Assist. Reprod. Genet. 28, 425–432 (2011).
Winkle, T., Rosenbusch, B., Gagsteiger, F., Paiss, T. & Zoller, N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J. Assist Reprod. Genet. 26, 41–46 (2009).
Walter, C. A., Intano, G. W., McCarrey, J. R., McMahan, C. A. & Walter, R. B. Mutation frequency declines during spermatogenesis in young mice but increases in old mice. Proc. Natl Acad. Sci. USA 95, 10015–10019 (1998).
Lowe, X. et al. Aneuploidies and micronuclei in the germ cells of male mice of advanced age. Mutat. Res. 338, 59–76 (1995).
Xiao, Y., Tates, A. D., Boei, J. & Natarajan, A. T. Aging and diethylstilbestrol-induced aneuploidy in male germ cells: a transgenic mouse model. Chromosoma 107, 507–513 (1998).
Miething, A. Arrested germ cell divisions in the ageing human testis. Andrologia 37, 10–16 (2005).
Allen, J. W., Collins, B. W. & Setzer, R. W. Spermatid micronucleus analysis of aging effects in hamsters. Mutat. Res. 316, 261–266 (1996).
Cabelof, D. C. et al. Attenuation of DNA polymerase beta-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat. Res. 500, 135–145 (2002).
Intano, G. W. et al. Base excision repair is limited by different proteins in male germ cell nuclear extracts prepared from young and old mice. Mol. Cell Biol. 22, 2410–2418 (2002).
Paul, C., Nagano, M. & Robaire, B. Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the Brown Norway rat. Biol. Reprod. 85, 1269–1278 (2011).
El-Domyati, M. M. et al. Deoxyribonucleic acid repair and apoptosis in testicular germ cells of aging fertile men: the role of the poly(adenosine diphosphate-ribosyl)ation pathway. Fertil. Steril. 91, 2221–2229 (2009).
Ren, K. & Pena de Ortiz, S. Non-homologous DNA end joining in the mature rat brain. J. Neurochem. 80, 949–959 (2002).
Vyjayanti, V. N. & Rao, K. S. DNA double strand break repair in brain: reduced NHEJ activity in aging rat neurons. Neurosci. Lett. 393, 18–22 (2006).
Um, J. H. et al. Tissue-specific changes of DNA repair protein Ku and mtHSP70 in aging rats and their retardation by caloric restriction. Mech. Ageing Dev. 124, 967–975 (2003).
Zheng, P., Schramm, R. D. & Latham, K. E. Developmental regulation and in vitro culture effects on expression of DNA repair and cell cycle checkpoint control genes in rhesus monkey oocytes and embryos. Biol. Reprod. 72, 1359–1369 (2005).
Twigg, J. P., Irvine, D. S. & Aitken, R. J. Oxidative damage to DNA in human spermatozoa does not preclude pronucleus formation at intracytoplasmic sperm injection. Hum. Reprod. 13, 1864–1871 (1998).
Nasr-Esfahani, M. H. et al. Effect of sperm DNA damage and sperm protamine deficiency on fertilization and embryo development post-ICSI. Reprod. Biomed. Online 11, 198–205 (2005).
Dimitriadis, F. et al. Effects of primary testicular damage on sperm DNA oxidative status and embryonic and foetal development. Andrologia 41, 282–296 (2009).
Fernandez-Gonzalez, R. et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol. Reprod. 78, 761–772 (2008).
Garcia-Palomares, S. et al. Long-term effects of delayed fatherhood in mice on postnatal development and behavioral traits of offspring. Biol. Reprod. 80, 337–342 (2009).
de La Rochebrochard, E. & Thonneau, P. Paternal age >or=40 years: an important risk factor for infertility. Am. J. Obstet. Gynecol. 189, 901–905 (2003).
Kleinhaus, K. et al. Paternal age and spontaneous abortion. Obstet. Gynecol. 108, 369–377 (2006).
Astolfi, P., De Pasquale, A. & Zonta, L. A. Late paternity and stillbirth risk. Hum. Reprod. 19, 2497–2501 (2004).
Martin, J. A. et al. Births: final data for 2009. Natl Vital Stat. Rep. 60, 1–70 (2011).
Gosden, R. G., Trasler, J., Lucifero, D. & Faddy, M. J. Rare congenital disorders, imprinted genes and assisted reproductive technology. Lancet 361, 1975–1977 (2003).
Frattarelli, J. L., Miller, K. A., Miller, B. T., Elkind-Hirsch, K. & Scott, R. T. Jr. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil. Steril. 90, 93–103 (2007).
Borini, A. et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 21, 2876–2881 (2006).
Serre, V. & Robaire, B. Paternal age affects fertility and progeny outcome in the Brown Norway rat. Fertil. Steril. 70, 625–631 (1998).
Crow, J. Spontaneous mutation as a risk factor. Exp. Clin. Immunogenet. 12, 121–128 (1995).
Crow, J. F. The origins, patterns and implications of human spontaneous mutation. Nat. Rev. Genet. 1, 40–47 (2000).
Goriely, A. & Wilkie, A. O. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am. J. Hum. Genet. 90, 175–200 (2012).
Hemminki, K. & Kyyronen, P. Parental age and risk of sporadic and familial cancer in offspring: implications for germ cell mutagenesis. Epidemiology 10, 747–751 (1999).
Hemminki, K., Kyyronen, P. & Vaittinen, P. Parental age as a risk factor of childhood leukemia and brain cancer in offspring. Epidemiology 10, 271–275 (1999).
Tolarova, M. M., Harris, J. A., Ordway, D. E. & Vargervik, K. Birth prevalence, mutation rate, sex ratio, parents' age, and ethnicity in Apert syndrome. Am. J. Med. Genet. 72, 394–398 (1997).
Rolf, C. & Nieschlag, E. Reproductive functions, fertility and genetic risks of ageing men. Exp. Clin. Endocrinol. Diabetes 109, 68–74 (2001).
Toriello, H. V. & Meck, J. M. Statement on guidance for genetic counseling in advanced paternal age. Genet. Med. 10, 457–460 (2008).
Penrose, L. S. Parental age and mutation. Lancet 269, 312–313 (1955).
Frans, E. M. et al. Advancing paternal age and bipolar disorder. Arch. Gen. Psychiatry 65, 1034–1040 (2008).
Grigoroiu-Serbanescu, M. et al. Paternal age effect on age of onset in bipolar I disorder is mediated by sex and family history. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 159, 567–579 (2012).
Flatscher-Bader, T. et al. Increased de novo copy number variants in the offspring of older males. Transl. Psychiatry 1, e34 (2011).
Kong, A. et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488, 471–475 (2012).
Dalman, C. & Allebeck, P. Paternal age and schizophrenia: further support for an association. Am. J. Psychiatry 159, 1591–1592 (2002).
Bertram, L. et al. Paternal age is a risk factor for Alzheimer disease in the absence of a major gene. Neurogenetics 1, 277–280 (1998).
Olshan, A. F., Schnitzer, P. G. & Baird, P. A. Paternal age and the risk of congenital heart defects. Teratology 50, 80–84 (1994).
Green, R. F. et al. Association of paternal age and risk for major congenital anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann. Epidemiol. 20, 241–249 (2010).
Smith, R. G. et al. Advancing paternal age is associated with deficits in social and exploratory behaviors in the offspring: a mouse model. PLoS One 4, e8456 (2009).
Foldi, C. J., Eyles, D. W., McGrath, J. J. & Burne, T. H. Advanced paternal age is associated with alterations in discrete behavioural domains and cortical neuroanatomy of C57BL/6J mice. Eur. J. Neurosci. 31, 556–564 (2010).
Saha, S., Barnett, A. G., Buka, S. L. & McGrath, J. J. Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr. Res. 115, 130–135 (2009).
Gavrilov, L. A. & Gavrilova, N. S. When should fatherhood stop? Science 277, 17–18 (1997).
Gavrilov, L. A. et al. Mutation load and human longevity. Mutat. Res. 377, 61–62 (1997).
Crow, J. F. Age and sex effects on human mutation rates: an old problem with new complexities. J. Radiat. Res. 47 (Suppl. B), B75–B82 (2006).
Eisenberg, D. T., Hayes, M. G. & Kuzawa, C. W. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc. Natl Acad. Sci. USA 109, 10251–10256 (2012).
Kimura, M. et al. Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 4, e37 (2008).
Goriely, A. et al. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat. Genet. 41, 1247–1252 (2009).
Orioli, I. M., Castilla, E. E., Scarano, G. & Mastroiacovo, P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. Am. J. Med. Genet. 59, 209–217 (1995).
Glaser, R. L. et al. Paternal origin of FGFR2 mutations in sporadic cases of Crouzon syndrome and Pfeiffer syndrome. Am. J. Hum. Genet. 66, 768–777 (2000).
Murdoch, J. L., Walker, B. A. & McKusick, V. A. Parental age effects on the occurrence of new mutations for the Marfan syndrome. Ann. Hum. Genet. 35, 331–336 (1972).
Schuffenecker, I. et al. Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma. Le Groupe d'Etude des Tumeurs a Calcitonine. Am. J. Hum. Genet. 60, 233–237 (1997).
Carlson, K. M. et al. Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am. J. Hum. Genet. 55, 1076–1082 (1994).
Choi, J. Y. et al. Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer 5, 143 (2005).
Author information
Authors and Affiliations
Contributions
Both authors contributed towards researching, writing, discussing, and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Paul, C., Robaire, B. Ageing of the male germ line. Nat Rev Urol 10, 227–234 (2013). https://doi.org/10.1038/nrurol.2013.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2013.18
This article is cited by
-
Meta-analysis shows no consistent evidence for senescence in ejaculate traits across animals
Nature Communications (2024)
-
Increased double-strand breaks in aged mouse male germ cells may result from changed expression of the genes essential for homologous recombination or nonhomologous end joining repair
Histochemistry and Cell Biology (2023)
-
High-resolution analyses of human sperm dynamic methylome reveal thousands of novel age-related epigenetic alterations
Clinical Epigenetics (2020)
-
Transcriptomic evidence that insulin signalling pathway regulates the ageing of subterranean termite castes
Scientific Reports (2020)
-
Insights into replicative senescence of human testicular peritubular cells
Scientific Reports (2019)