Abstract
The amyloid hypothesis has yielded a series of well-validated candidate drug targets with potential for the treatment of Alzheimer disease (AD). Three proteases that are involved in the processing of amyloid precursor protein—α-secretase, β-secretase and γ-secretase—are of particular interest as they are central to the generation and modulation of amyloid-β peptide and can be targeted by small compounds in vitro and in vivo. Given that these proteases also fulfill other important biological roles, inhibiting their activity will clearly be inherently associated with mechanism-based toxicity. Carefully determining a suitable therapeutic window and optimizing the selectivity of the drug treatments towards amyloid precursor protein processing might be ways of overcoming this potential complication. Secretase inhibitors are likely to be the first small-molecule therapies aimed at AD modification that will be fully tested in the clinic. Success or failure of these first-generation AD therapies will have enormous consequences for further drug development efforts for AD and possibly other neurodegenerative conditions.
Key Points
-
α-Secretase, β-secretase and γ-secretase are proteases that control the production of amyloid-β (Aβ) in the brain
-
The secretases represent the most promising drug targets for Alzheimer disease therapies
-
The α-secretase activity is mediated by a series of membrane-bound proteases, further research is needed to identify which of these proteases are most important for processing amyloid precursor protein
-
β-Secretase and γ-secretase research has progressed enormously and compounds designed to attenuate their activity are in clinic trials
-
The current trials test only whether elimination or attenuating Aβ in moderate to advanced AD could be beneficial
-
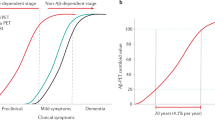
A real test of the amyloid hypothesis will require drug testing at earlier stages of the disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Knopman, D. S. Mediterranean diet and late-life cognitive impairment: a taste of benefit. JAMA 302, 686–687 (2009).
Lazarov, O. et al. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell 120, 701–713 (2005).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Reinhard, C., Hebert, S. S. & De Strooper, B. The amyloid-β precursor protein: integrating structure with biological function. EMBO J. 24, 3996–4006 (2005).
Sisodia, S. S., Koo, E. H., Beyreuther, K., Unterbeck, A. & Price, D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science 248, 492–495 (1990).
Weidemann, A. et al. Proteolytic processing of the Alzheimer's disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases. J. Biol. Chem. 274, 5823–5829 (1999).
Seubert, P. et al. Secretion of β-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature 361, 260–263 (1993).
Golde, T. E., Estus, S., Younkin, L. H., Selkoe, D. J. & Younkin, S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science 255, 728–730 (1992).
Haass, C., Koo, E. H., Mellon, A., Hung, A. Y. & Selkoe, D. J. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357, 500–503 (1992).
De Strooper, B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998).
Haass, C. & Selkoe, D. J. Cellular processing of β-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75, 1039–1042 (1993).
Hung, A. Y. et al. Activation of protein kinase C inhibits cellular production of the amyloid β-protein. J. Biol. Chem. 268, 22959–22962 (1993).
Skovronsky, D. M., Moore, D. B., Milla, M. E., Doms, R. W. & Lee, V. M. Protein kinase C-dependent α-secretase competes with β-secretase for cleavage of amyloid-β precursor protein in the trans-golgi network. J. Biol. Chem. 275, 2568–2575 (2000).
Rossner, S. et al. Constitutive overactivation of protein kinase C in guinea pig brain increases α-secretory APP processing without decreasing β-amyloid generation. Eur. J. Neurosci. 12, 3191–3200 (2000).
Gowing, E. et al. Chemical characterization of Aβ 17–42 peptide, a component of diffuse amyloid deposits of Alzheimer disease. J. Biol. Chem. 269, 10987–10990 (1994).
Higgins, L. S., Murphy, G. M. Jr, Forno, L. S., Catalano, R. & Cordell, B. P3 beta-amyloid peptide has a unique and potentially pathogenic immunohistochemical profile in Alzheimer's disease brain. Am. J. Pathol. 149, 585–596 (1996).
Ring, S. et al. The secreted β-amyloid precursor protein ectodomain APPsα is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 27, 7817–7826 (2007).
Lammich, S. et al. Constitutive and regulated α-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl Acad. Sci. USA 96, 3922–3927 (1999).
Postina, R. et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 113, 1456–1464 (2004).
Asai, M. et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP α-secretase. Biochem. Biophys. Res. Commun. 301, 231–235 (2003).
Buxbaum, J. D. et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 273, 27765–27767 (1998).
Koike, H. et al. Membrane-anchored metalloprotease MDC9 has an α-secretase activity responsible for processing the amyloid precursor protein. Biochem. J. 343, 371–375 (1999).
Tanabe, C. et al. ADAM19 is tightly associated with constitutive Alzheimer's disease APP α-secretase in A172 cells. Biochem. Biophys. Res. Commun. 352, 111–117 (2007).
Farzan, M., Schnitzler, C. E., Vasilieva, N., Leung, D. & Choe, H. BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc. Natl Acad. Sci. USA 97, 9712–9717 (2000).
Yan, R., Munzner, J. B., Shuck, M. E. & Bienkowski, M. J. BACE2 functions as an alternative α-secretase in cells. J. Biol. Chem. 276, 34019–34027 (2001).
Weskamp, G. et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol. Cell. Biol. 22, 1537–1544 (2002).
Hartmann, D. et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for α-secretase activity in fibroblasts. Hum. Mol. Genet. 11, 2615–2624 (2002).
Black, R. A. et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385, 729–733 (1997).
Maretzky, T. et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and β-catenin translocation. Proc. Natl Acad. Sci. USA 102, 9182–9187 (2005).
Reiss, K. et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and β-catenin nuclear signalling. EMBO J. 24, 742–752 (2005).
Allinson, T. M., Parkin, E. T., Turner, A. J. & Hooper, N. M. ADAMs family members as amyloid precursor protein α-secretases. J. Neurosci. Res. 74, 342–352 (2003).
Bandyopadhyay, S., Goldstein, L. E., Lahiri, D. K. & Rogers, J. T. Role of the APP non-amyloidogenic signaling pathway and targeting α-secretase as an alternative drug target for treatment of Alzheimer's disease. Curr. Med. Chem. 14, 2848–2864 (2007).
Tippmann, F., Hundt, J., Schneider, A., Endres, K. & Fahrenholz, F. Up-regulation of the α-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 6, 1643–1654 (2009).
Caccamo, A. et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 49, 671–682 (2006).
Wolf, B. A. et al. Muscarinic regulation of Alzheimer's disease amyloid precursor protein secretion and amyloid β-protein production in human neuronal NT2N cells. J. Biol. Chem. 270, 4916–4922 (1995).
Zimmermann, M. et al. Acetylcholinesterase inhibitors increase ADAM10 activity by promoting its trafficking in neuroblastoma cell lines. J. Neurochem. 90, 1489–1499 (2004).
Hussain, I. et al. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol. Cell. Neurosci. 14, 419–427 (1999).
Vassar, R. et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999).
Yan, R. et al. Membrane-anchored aspartyl protease with Alzheimer's disease β-secretase activity. Nature 402, 533–537 (1999).
Sinha, S. et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 402, 537–540 (1999).
Lin, X. et al. Human aspartic protease memapsin 2 cleaves the β-secretase site of β-amyloid precursor protein. Proc. Natl Acad. Sci. USA 97, 1456–1460 (2000).
Cole, S. L. & Vassar, R. The role of amyloid precursor protein processing by BACE1, the β-secretase, in Alzheimer disease pathophysiology. J. Biol. Chem. 283, 29621–29625 (2008).
Luo, Y. et al. Mice deficient in BACE1, the Alzheimer's β-secretase, have normal phenotype and abolished β-amyloid generation. Nat. Neurosci. 4, 231–232 (2001).
Cai, H. et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 4, 233–234 (2001).
Roberds, S. L. et al. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol. Genet. 10, 1317–1324 (2001).
Dominguez, D. et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J. Biol. Chem. 280, 30797–30806 (2005).
Ohno, M. et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron 41, 27–33 (2004).
Laird, F. M. et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-β amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 25, 11693–11709 (2005).
McConlogue, L. et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J. Biol. Chem. 282, 26326–26334 (2007).
Nishitomi, K. et al. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J. Neurochem. 99, 1555–1563 (2006).
Singer, O. et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat. Neurosci. 8, 1343–1349 (2005).
Yan, R., Han, P., Miao, H., Greengard, P. & Xu, H. The transmembrane domain of the Alzheimer's β-secretase (BACE1) determines its late Golgi localization and access to β-amyloid precursor protein (APP) substrate. J. Biol. Chem. 276, 36788–36796 (2001).
Kitazume, S. et al. Alzheimer's β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc. Natl Acad. Sci. USA 98, 13554–13559 (2001).
Lichtenthaler, S. F. et al. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J. Biol. Chem. 278, 48713–48719 (2003).
Li, Q. & Sudhof, T. C. Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE 1. J. Biol. Chem. 279, 10542–10550 (2004).
Pastorino, L. et al. BACE (β-secretase) modulates the processing of APLP2 in vivo. Mol. Cell. Neurosci. 25, 642–649 (2004).
Eggert, S. et al. The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves α-, β-, γ-, and ε-like cleavages: modulation of APLP-1 processing by n-glycosylation. J. Biol. Chem. 279, 18146–18156 (2004).
von Arnim, C. A. et al. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J. Biol. Chem. 280, 17777–17785 (2005).
Wong, H. K. et al. β Subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J. Biol. Chem. 280, 23009–23017 (2005).
Kim, D.Y. et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat. Cell Biol. 9, 755–764 (2007).
Hu, X. et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 22, 2970–2980 (2008).
Willem, M. et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science 314, 664–666 (2006).
Hu, X et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520–1525 (2006).
Silvestri, R. Boom in the development of non-peptidic β-secretase (BACE1) inhibitors for the treatment of Alzheimer's disease. Med. Res. Rev. 29, 295–338 (2009).
Hills, I. D. & Vacca, J. P. Progress toward a practical BACE-1 inhibitor. Curr. Opin. Drug Discov. Devel. 10, 383–391 (2007).
Rajendran, L. et al. Efficient inhibition of the Alzheimer's disease β-secretase by membrane targeting. Science 320, 520–523 (2008).
De Strooper, B. Aph-1, Pen-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron 38, 9–12 (2003).
Wolfe, M. S. & Kopan, R. Intramembrane proteolysis: theme and variations. Science 305, 1119–1123 (2004).
Takasugi, N. et al. The role of presenilin cofactors in the γ-secretase complex. Nature 422, 438–441 (2003).
Edbauer, D. et al. Reconstitution of γ-secretase activity. Nat. Cell Biol. 5, 486–488 (2003).
Shah, S. et al. Nicastrin functions as a γ-secretase-substrate receptor. Cell 122, 435–447 (2005).
Chavez-Gutierrez, L. et al. Glu(332) in the nicastrin ectodomain is essential for γ-secretase complex maturation but not for its activity. J. Biol. Chem. 283, 20096–20105 (2008).
Hébert, S. S. et al. Coordinated and widespread expression of γ-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol. Dis. 17, 260–272 (2004).
Shirotani, K., Edbauer, D., Prokop, S., Haass, C. & Steiner, H. Identification of distinct γ-secretase complexes with different APH-1 variants. J. Biol. Chem. 279, 41340–41345 (2004).
Ma, G., Li, T., Price, D. L. & Wong, P. C. APH-1a is the principal mammalian APH-1 isoform present in γ-secretase complexes during embryonic development. J. Neurosci. 25, 192–198 (2005).
Serneels, L. et al. Differential contribution of the three Aph1 genes to γ-secretase activity in vivo. Proc. Natl Acad. Sci. USA 102, 1719–1724 (2005).
Serneels, L. et al. γ-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science 324, 639–642 (2009).
Wolfe, M. S. et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517 (1999).
Osenkowski, P. et al. Cryoelectron microscopy structure of purified γ-secretase at 12 A resolution. J. Mol. Biol. 385, 642–652 (2009).
Lazarov, V. K. et al. Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores. Proc. Natl Acad. Sci. USA 103, 6889–6894 (2006).
Tolia, A., Chavez-Gutierrez, L. & De Strooper, B. Contribution of presenilin transmembrane domains 6 and 7 to a water-containing cavity in the γ-secretase complex. J. Biol. Chem. 281, 27633–27642 (2006).
Sato, C., Morohashi, Y., Tomita, T. & Iwatsubo, T. Structure of the catalytic pore of γ-secretase probed by the accessibility of substituted cysteines. J. Neurosci. 26, 12081–12088 (2006).
Kopan, R. & Ilagan, M. X. γ-Secretase: proteasome of the membrane? Nat. Rev. Mol. Cell Biol. 5, 499–504 (2004).
Kakuda, N. et al. Equimolar production of amyloid β-protein and amyloid precursor protein intracellular domain from β-carboxyl-terminal fragment by γ-secretase. J. Biol. Chem. 281, 14776–14786 (2006).
Golde, T. E., Eckman, C. B. & Younkin, S. G. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim. Biophys. Acta 1502, 172–187 (2000).
Jarrett, J. T., Berger, E. P. & Lansbury, P. T. Jr. The carboxy terminus of β amyloid protein is critical for the seeding of amyloid formation: Implications for pathogenesis of Alzheimer's disease. Biochemistry 32, 4693–4697 (1993).
McGowan, E. et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47, 191–199 (2005).
Wang, R., Wang, B., He, W. & Zheng, H. Wild-type presenilin 1 protects against Alzheimer's disease mutation-induced amyloid pathology. J. Biol. Chem. 281, 15330–15336 (2006).
Kim, J. et al. Aβ40 inhibits amyloid deposition in vivo. J. Neurosci. 27, 627–633 (2007).
Wolfe, M. S. Inhibition and modulation of γ-secretase for Alzheimer's disease. Neurotherapeutics 5, 391–398 (2008).
Bateman, R. J. et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 66, 48–54 (2009).
Siemers, E. R. et al. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-β after inhibition of γ-secretase. Clin. Neuropharmacol. 30, 317–325 (2007).
Dovey, H. F. et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 76, 173–181 (2001).
Abramowski, D. et al. Dynamics of Aβ turnover and deposition in different β-amyloid precursor protein transgenic mouse models following γ-secretase inhibition. J. Pharmacol. Exp. Ther. 327, 411–424 (2008).
De Strooper, B. et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999).
Wong, P. C. et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387, 288–292 (1997).
Shen, J. et al. Skeletal and CNS defects in presenilin-1-deficient mice. Cell 89, 629–639 (1997).
Searfoss, G. H. et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J. Biol. Chem. 278, 46107–46116 (2003).
Wong, G. T. et al. Chronic treatment with the γ-secretase inhibitor LY-411, 575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 279, 12876–12882 (2004).
Li, T. et al. Epidermal growth factor receptor and notch pathways participate in the tumor suppressor function of γ-secretase. J. Biol. Chem. 282, 32264–32273 (2007).
Yankner, B. A. et al. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science 245, 417–420 (1989).
Siemers, E. R. et al. Effects of a γ-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology 66, 602–604 (2006).
Fraering, P. C. et al. γ-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide-binding site. J. Biol. Chem. 280, 41987–41996 (2005).
Netzer, W. J. et al. Gleevec inhibits β-amyloid production but not Notch cleavage. Proc. Natl Acad. Sci. USA 100, 12444–12449 (2003).
Fagan, T. DC: New γ-secretase inhibitors hit APP, spare Notch. The AlzGene Database. Alzheimer Research Forum [online], (2008).
Mayer, S. C. et al. Discovery of begacestat, a Notch-1-sparing γ-secretase inhibitor for the treatment of Alzheimer's disease. J. Med. Chem. 51, 7348–7351 (2008).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Kukar, T. & Golde, T. E. Possible mechanisms of action of NSAIDs and related compounds that modulate γ-secretase cleavage. Curr. Top. Med. Chem. 8, 47–53 (2008).
Kukar, T. et al. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Aβ42 production. Nat. Med. 11, 545–550 (2005).
Weggen, S. et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid β42 production by direct modulation of γ-secretase activity. J. Biol. Chem. 278, 31831–31837 (2003).
Green, R. C. et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 302, 2557–2564 (2009).
Kukar, T. L. et al. Substrate-targeting γ-secretase modulators. Nature 453, 925–929 (2008).
Pissarnitski, D. Advances in gamma-secretase modulation. Curr. Opin. Drug Discov. Devel. 10, 392–402 (2007).
Klunk, W. E. & Mathis, C. A. The future of amyloid-beta imaging: a tale of radionuclides and tracer proliferation. Curr. Opin. Neurol. 21, 683–687 (2008).
Acknowledgements
This work was supported by a NIH/NIA grant (number AG25531) to T. Golde. B. De Strooper was supported by an Artificial SynApse (IWT-ASAP) grant, the Federal Office for Scientific Affairs, Belgium (IUAP P6/43), a Methusalem grant from the Flemish Government and a grant from the European Union (MEMOSAD, F2-2007-200611). R. Vassar received support from the NIH (grant numbers: P01 AG021184, R01 AG022560 and R01 AG030142), the Alzheimer's Association and the MetLife Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
B. De Strooper has received honoraria from Eisai, Eli Lilly, EnVivo, Johnson & Johnson and Probiodrug for consulting and has received research funding from Eli Lilly, EnVivo and Johnson & Johnson. He also holds the following European patent (EP) applications (EP 00200671.6, EP 01201015.3, EP 01202228.1, EP 02078915.2, EP 04106516.0, EP 05107454.0, EP 06120346.9, EP 07106482.8, EP 08063269, EP 09053985) with the Flanders institute for Biotechnology. T. Golde holds one patent application (WO0178721) with the Mayo Clinic. R. Vasser declares no competing interests.
Rights and permissions
About this article
Cite this article
De Strooper, B., Vassar, R. & Golde, T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol 6, 99–107 (2010). https://doi.org/10.1038/nrneurol.2009.218
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2009.218
This article is cited by
-
Mitigating alcohol-induced neurohepatotoxicity in male albino rats with avocado and mustard
Journal of Umm Al-Qura University for Applied Sciences (2024)
-
Peptide aptamer targeting Aβ–PrP–Fyn axis reduces Alzheimer’s disease pathologies in 5XFAD transgenic mouse model
Cellular and Molecular Life Sciences (2023)
-
Alzheimer’s disease – the journey of a healthy brain into organ failure
Molecular Neurodegeneration (2022)
-
Titanium dioxide and carbon black nanoparticles disrupt neuronal homeostasis via excessive activation of cellular prion protein signaling
Particle and Fibre Toxicology (2022)
-
γ-Secretase in Alzheimer’s disease
Experimental & Molecular Medicine (2022)