Key Points
-
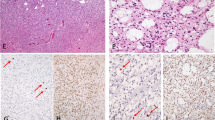
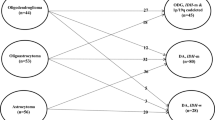
In the 2016 WHO classification of brain tumours, diffuse gliomas — including grade II gliomas — are defined by both morphological and molecular criteria
-
Prognosis is more closely associated with molecular diagnosis than with morphology, but grade remains prognostically important
-
Immunohistochemistry and cytogenetics provide an accurate diagnosis for most patients, whereas chromosomal and gene arrays provide more complete diagnostic information for some tumours
-
Total resection of all tumour that is visible on MRI without inflicting additional neurological deficit is most common in patients with isocitrate dehydrogenase (IDH)-mutated tumours, and results in increased survival in these patients
-
Although radiation alone and temozolomide alone seem to result in comparable progression-free survival overall in patients with grade II glioma, those with IDH-mutated tumours without 1p/19q codeletion have longer progression-free survival with radiation therapy than with temozolomide
-
Treatment with radiation therapy plus chemotherapy with procarbazine, lomustine and vincristine results in prolongation of survival in most patients, especially those with oligodendroglioma or other IDH-mutated tumours
Abstract
Diffuse WHO grade II gliomas are histologically and genetically heterogeneous. The 2016 WHO classification redefines grade II gliomas with respect to morphological and molecular tumour alterations: grade II oligodendrogliomas are defined by the presence of whole-arm codeletion in chromosomal arms 1p/19q, whereas isocitrate dehydrogenase (IDH) mutations define subclasses of astrocytoma. Although histological grade remains useful, the prognoses of patients with glioma are more tightly associated with molecular alterations than with grade, and chromosomal and gene array technologies are becoming increasingly beneficial in understanding tumour genetic heterogeneity. The indolent nature of the disease often creates subtle neurological symptoms that can be overlooked or misunderstood, resulting in delayed diagnosis. Seizures often herald the diagnosis, especially in patients who have IDH mutations, which are associated with an increased production of 2-hydroxyglutarate. Treatment paradigms have shifted, owing to new diagnostic criteria and new clinical trial evidence. Patients benefit more from chemoradiation than radiation alone, especially those with tumour IDH1 Arg132His mutations; gross total resection of the tumour, including tumours with IDH mutations, is associated with prolonged survival. Initial observation remains appropriate in patients whose rate of disease growth is not yet completely defined; such patients could include those with completely resected disease and those with 1p/19q codeleted tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
International Agency for Research on Cancer. WHO Classification of Tumours of the Central Nervous System 4 edn Vol. 1 ( eds Louis, D. N., Ohgaki, H., Wiestler, O. D. & Cavenee, W. K. ) (WHO/IARC, 2016).
Reuss, D. E. et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 130, 407–417 (2015).
Cancer Genome Atlas Research Network et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481–2498 (2015).
Jenkins, R. B. et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 66, 9852–9861 (2006).
Eckel-Passow, J. E. et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N. Engl. J. Med. 372, 2499–2508 (2015).
Baumert, B. G. et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 17, 1521–1532 (2016).
Buckner, J. C. et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N. Engl. J. Med. 374, 1344–1355 (2016).
Leeper, H. E. et al. IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas. Oncotarget 6, 30295–30305 (2015).
Ceccarelli, M. et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164, 550–563 (2016).
Stacey, S. N. et al. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat. Genet. 43, 1098–1103 (2011).
Shete, S. et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat. Genet. 41, 899–904 (2009).
Sanson, M. et al. Chromosome 7p11.2 (EGFR) variation influences glioma risk. Hum. Mol. Genet. 20, 2897–2904 (2011).
Walsh, K. M. et al. Analysis of 60 reported glioma risk SNPs replicates published GWAS findings but fails to replicate associations from published candidate-gene studies. Genet. Epidemiol. 37, 222–228 (2013).
Jenkins, R. B. et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat. Genet. 44, 1122–1125 (2012).
Kinnersley, B. et al. Genome-wide association study identifies multiple susceptibility loci for glioma. Nat. Commun. 6, 8559 (2015).
Rice, T. et al. Understanding inherited genetic risk of adult glioma — a review. Neurooncol. Pract. 3, 10–16 (2016).
Duffau, H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 13, 885–897 (2006).
Pascual-Leone, A., Amedi, A., Fregni, F. & Merabet, L. B. The plastic human brain cortex. Annu. Rev. Neurosci. 28, 377–401 (2005).
Martinet, L. E., Ahmed, O. J., Lepage, K. Q., Cash, S. S. & Kramer, M. A. Slow spatial recruitment of neocortex during secondarily generalized seizures and its relation to surgical outcome. J. Neurosci. 35, 9477–9490 (2015).
Yang, Y. et al. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J. Clin. Neurosci. 31, 56–62 (2016).
Huberfeld, G. & Vecht, C. J. Seizures and gliomas — towards a single therapeutic approach. Nat. Rev. Neurol. 12, 204–216 (2016).
Koekkoek, J. A. et al. Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro Oncol. 17, 924–934 (2015).
Englot, D. J., Han, S. J., Berger, M. S., Barbaro, N. M. & Chang, E. F. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery 70, 921–928 (2012).
Duffau, H. et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J. Neurol. Neurosurg. Psychiatry 74, 901–907 (2003).
Ragel, B. T. et al. The role of biopsy in the management of patients with presumed diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 125, 481–501 (2015).
Aghi, M. K. et al. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 125, 503–530 (2015).
Berger, M. S., Deliganis, A. V., Dobbins, J. & Keles, G. E. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74, 1784–1791 (1994).
Castellano, A. et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol. 14, 192–202 (2012).
Sanai, N., Polley, M. Y. & Berger, M. S. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J. Neurosurg. 112, 1–9 (2010).
Smith, J. S. et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 26, 1338–1345 (2008).
Kizilbash, S. H. et al. The impact of concurrent temozolomide with adjuvant radiation and IDH mutation status among patients with anaplastic astrocytoma. J. Neurooncol. 120, 85–93 (2014).
Beiko, J. et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 16, 81–91 (2014).
Laack, N. N. et al. Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int. J. Radiat. Oncol. Biol. Phys. 63, 1175–1183 (2005).
Douw, L. et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 8, 810–818 (2009).
Shaw, E. et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J. Clin. Oncol. 20, 2267–2276 (2002).
Karim, A. B. et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 36, 549–556 (1996).
Fisher, B. J. et al. A phase II study of a temozolomide-based chemoradiotherapy regimen for high-risk low-grade gliomas: preliminary results of RTOG 0424 [abstract]. J. Clin. Oncol. 31 (Suppl.), 2008 (2013).
Ryken, T. C., Parney, I., Buatti, J., Kalkanis, S. N. & Olson, J. J. The role of radiotherapy in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 125, 551–583 (2015).
Gondi, V., Hermann, B. P., Mehta, M. P. & Tome, W. A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 85, 348–354 (2013).
Shih, H. A. et al. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer 121, 1712–1719 (2015).
Kazda, T. et al. Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat. Oncol. 9, 139 (2014).
Mason, W. P., Krol, G. S. & DeAngelis, L. M. Low-grade oligodendroglioma responds to chemotherapy. Neurology 46, 203–207 (1996).
Buckner, J. C. et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J. Clin. Oncol. 21, 251–255 (2003).
Stege, E. M. et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer 103, 802–809 (2005).
Hoang-Xuan, K. et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J. Clin. Oncol. 22, 3133–3138 (2004).
Brada, M. et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann. Oncol. 14, 1715–1721 (2003).
Pace, A. et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann. Oncol. 14, 1722–1726 (2003).
Quinn, J. A. et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J. Clin. Oncol. 21, 646–651 (2003).
Jaeckle, K. et al. ATCT-16CODEL (ALLIANCE-N0577; EORTC-26081/2208; NRG-1071; NCIC-CEC-2): phase III randomized study of RT versus RT + TMZ versus TMZ for newly diagnosed 1p/19q-codeleted anaplastic glioma. Analysis of patients treated on the original protocol design. Neuro Oncol. 17 (Suppl. 5), v4–v5 (2015).
Johnson, B. E. et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343, 189–193 (2014).
van den Bent, M. J. et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366, 985–990 (2005).
Shaw, E. G. et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J. Neurosurg. 109, 835–841 (2008).
Buckner, J. et al. ATCT-09IDH1 R132H mutations in NRG oncology/RTOG 9802: phase III study of radiation therapy (RT) alone versus RT plus procarbazine, CCNU, and vincristine (PCV) in patients with low grade glioma (LGG). Neuro Oncol. 17 (Suppl. 5), v3 (2015).
Cairncross, J. G. et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 32, 783–790 (2014).
van den Bent, M. J. et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 31, 344–350 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00887146?term=n0577&rank=1 (2017).
Buckner, J. et al. AT-13R9802: phase III study of radiation therapy (RT) with or without procarbazine, CCNU, and vincristine (PCV) in low-grade qlioma: results by histologic type. Neuro Oncol. 16 (Suppl. 5), v11 (2014).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, substantial contribution to discussion of content and writing the article. J.B. reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Buckner, J., Giannini, C., Eckel-Passow, J. et al. Management of diffuse low-grade gliomas in adults — use of molecular diagnostics. Nat Rev Neurol 13, 340–351 (2017). https://doi.org/10.1038/nrneurol.2017.54
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.54
This article is cited by
-
Amide proton transfer weighted and diffusion weighted imaging based radiomics classification algorithm for predicting 1p/19q co-deletion status in low grade gliomas
BMC Medical Imaging (2024)
-
Prospective Identification of Prognostic Hot-Spot Mutant Gene Signatures for Leukemia: A Computational Study Based on Integrative Analysis of TCGA and cBioPortal Data
Molecular Biotechnology (2023)
-
Postoperative prognostic nomogram for adult grade II/III astrocytoma in the Chinese Han population
Health Information Science and Systems (2023)
-
The role of extent of resection on the prognosis of low-grade astrocytoma: a systematic review and meta-analysis
Egyptian Journal of Neurosurgery (2022)
-
Changes in clinical management of diffuse IDH-mutated lower-grade gliomas: patterns of care in a 15-year period
Journal of Neuro-Oncology (2022)