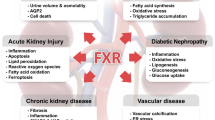
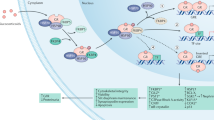
Abstract
Diabetes is the leading cause of end-stage renal disease in developed countries. In spite of glucose and blood pressure control, for example by use of angiotensin II receptor blockers, diabetic nephropathy still develops and progresses in affected patients and the development of additional protective therapeutic interventions is, therefore, required. Nuclear hormone receptors are transcription factors that regulate carbohydrate metabolism, lipid metabolism, the immune response, and inflammation. These receptors also modulate the development of fibrosis. As a result of their diverse biological effects, nuclear hormone receptors have become major pharmaceutical targets for the treatment of a host of diseases. The increasing prevalence of diabetic nephropathy has led intense investigation into the role that nuclear hormone receptors may have in slowing or preventing the progression of renal disease. This role of nuclear hormone receptors would be associated with improvements in metabolism, the immune response, and inflammation. Eight nuclear receptors have shown a renoprotective effect in the context of diabetic nephropathy. This Review discusses the evidence regarding the beneficial effects of the activation of these receptors in preventing the progression of diabetic nephropathy and describes how the discovery and development of compounds that modulate the activity of nuclear hormone receptors may provide potential additional therapeutic approaches in the management of diabetic nephropathy.
Key Points
-
Nuclear hormone receptor activity is abnormal in the diabetic kidney
-
Nuclear hormone receptors regulate metabolism, inflammation, oxidative stress and fibrosis
-
Modulation of the activity of the peroxisome-proliferator-associated receptor, vitamin D receptor, farnesoid X receptor and estrogen receptor holds great promise in the treatment of diabetic nephropathy
-
Further evidence on the role of nuclear hormone receptors in the diabetic kidney and the identification of additional specific ligands of these receptors will probably result in development of additional therapeutic strategies for diabetic nephropathy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mauer, S. M. Structural–functional correlations of diabetic nephropathy. Kidney Int. 45, 612–622 (1994).
Qian, Y., Feldman, E., Pennathur, S., Kretzler, M. & Brosius, F. C. 3rd. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57, 1439–1445 (2008).
Forbes, J. M., Coughlan, M. T. & Cooper, M. E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57, 1446–1454 (2008).
Zhu, Y., Usui, H. K. & Sharma, K. Regulation of transforming growth factor beta in diabetic nephropathy: implications for treatment. Semin. Nephrol. 27, 153–160 (2007).
Gurley, S. B. & Coffman, T. M. The renin–angiotensin system and diabetic nephropathy. Semin. Nephrol. 27, 144–152 (2007).
Weinberg, J. M. Lipotoxicity. Kidney Int. 70, 1560–1566 (2006).
Kimmelstiel, P. & Wilson, C. Intercapillary lesions in the glomeruli of the kidney. Am. J. Pathol. 12, 83–98.7 (1936).
Wilens, S. L. & Elster, S. K. The role of lipid deposition in renal arteriolar sclerosis. Am. J. Med. Sci. 219, 183–196 (1950).
Sun, L., Halaihel, N., Zhang, W., Rogers, T. & Levi, M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J. Biol. Chem. 277, 18919–18927 (2002).
Proctor, G. et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55, 2502–2509 (2006).
Jiang, T. et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J. Biol. Chem. 280, 32317–32325 (2005).
Wang, Z. et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54, 2328–2335 (2005).
Fried, L. F., Orchard, T. J. & Kasiske, B. L. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 59, 260–269 (2001).
Levi, M. Do statins have a beneficial effect on the kidney? Nat. Clin. Pract. Nephrol. 2, 666–667 (2006).
Evans, R. M. The steroid and thyroid hormone receptor superfamily. Science 240, 889–895 (1988).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Bookout, A. L. et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126, 789–799 (2006).
Guan, Y. & Breyer, M. D. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 60, 14–30 (2001).
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Niehof, M. & Borlak, J. HNF4 alpha and the Ca-channel TRPC1 are novel disease candidate genes in diabetic nephropathy. Diabetes 57, 1069–1077 (2008).
Chawla, A., Repa, J. J., Evans, R. M. & Mangelsdorf, D. J. Nuclear receptors and lipid physiology: opening the X-files. Science 294, 1866–1870 (2001).
Issemann, I. & Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347, 645–650 (1990).
Skogsberg, J. et al. Characterization of the human peroxisome proliferator activated receptor delta gene and its expression. Int. J. Mol. Med. 6, 73–81 (2000).
Forman, B. M., Chen, J. & Evans, R. M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl Acad. Sci. USA 94, 4312–4317 (1997).
Kliewer, S. A. et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 83, 813–819 (1995).
Willson, T. M., Lehmann, J. M. & Kliewer, S. A. Discovery of ligands for the nuclear peroxisome proliferator-activated receptors. Ann. NY Acad. Sci. 804, 276–283 (1996).
Schopfer, F. J. et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc. Natl Acad. Sci. USA 102, 2340–2345 (2005).
Lefebvre, P., Chinetti, G., Fruchart, J. C. & Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Invest. 116, 571–580 (2006).
Balakumar, P., Arora, M. K. & Singh, M. Emerging role of PPAR ligands in the management of diabetic nephropathy. Pharmacol. Res. 60, 170–173 (2009).
Delerive, P., Fruchart, J. C. & Staels, B. Peroxisome proliferator-activated receptors in inflammation control. J. Endocrinol. 169, 453–459 (2001).
Ouali, F., Djouadi, F., Merlet-Bénichou, C. & Bastin, J. Dietary lipids regulate beta-oxidation enzyme gene expression in the developing rat kidney. Am. J. Physiol. 275, F777–F784 (1998).
Shin, S. J. et al. Peroxisome proliferator-activated receptor-alpha activator fenofibrate prevents high-fat diet-induced renal lipotoxicity in spontaneously hypertensive rats. Hypertens. Res. 32, 835–845 (2009).
Portilla, D. et al. Etomoxir-induced PPARalpha-modulated enzymes protect during acute renal failure. Am. J. Physiol. Renal Physiol. 278, F667–F675 (2000).
Li, S. et al. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 76, 1049–1062 (2009).
Roman, R. J., Ma, Y. H., Frohlich, B. & Markham, B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension 21, 985–988 (1993).
Guan, Y., Zhang, Y., Davis, L. & Breyer, M. D. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am. J. Physiol. 273, F1013–F1022 (1997).
Kono, K. et al. PPAR{alpha} attenuates the proinflammatory response in activated mesangial cells. Am. J. Physiol. Renal Physiol. 296, F328–F336 (2009).
Kamijo, Y. et al. Peroxisome proliferator-activated receptor alpha protects against glomerulonephritis induced by long-term exposure to the plasticizer di-(2-ethylhexyl)phthalate. J. Am. Soc. Nephrol. 18, 176–188 (2007).
Park, C. W. et al. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes 55, 885–893 (2006).
Park, C. W. et al. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 69, 1511–1517 (2006).
Calkin, A. C. et al. PPAR-alpha and -gamma agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol. Dial. Transplant. 21, 2399–2405 (2006).
Chen, Y. J. & Quilley, J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J. Pharmacol. Exp. Ther. 324, 658–663 (2008).
Balakumar, P., Chakkarwar, V. A. & Singh, M. Ameliorative effect of combination of benfotiamine and fenofibrate in diabetes-induced vascular endothelial dysfunction and nephropathy in the rat. Mol. Cell. Biochem. 320, 149–162 (2009).
Nagai, T., Tomizawa, T., Nakajima, K. & Mori, M. Effect of bezafibrate or pravastatin on serum lipid levels and albuminuria in NIDDM patients. J. Atheroscler. Thromb. 7, 91–96 (2000).
Sacks, F. M. After the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: implications for fenofibrate. Am. J. Cardiol. 102, 34L–40L (2008).
Gonzalez, F. J. & Shah, Y. M. PPARα: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology 246, 2–8 (2008).
Ruan, X. Z. et al. PPAR agonists protect mesangial cells from interleukin 1beta-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J. Am. Soc. Nephrol. 14, 593–600 (2003).
Rigamonti, E., Chinetti-Gbaguidi, G. & Staels, B. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma, and LXRs in mice and men. Arterioscler. Thromb. Vasc. Biol. 28, 1050–1059 (2008).
Ruan, X., Zheng, F. & Guan, Y. PPARs and the kidney in metabolic syndrome. Am. J. Physiol. Renal Physiol. 294, F1032–F1047 (2008).
Yoshioka, S. et al. Antihypertensive effects of CS-045 treatment in obese Zucker rats. Metabolism 42, 75–80 (1993).
Sarafidis, P. A. & Bakris, G. L. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int. 70, 1223–1233 (2006).
Guan, Y. et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat. Med. 11, 861–866 (2005).
Carmona, M. C. et al. Fenofibrate prevents Rosiglitazone-induced body weight gain in ob/ob mice. Int. J. Obes. (Lond.) 29, 864–871 (2005).
Cha, D. R. et al. Peroxisome proliferator activated receptor alpha/gamma dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes 56, 2036–2045 (2007).
Wu, J. et al. Liver X receptor-alpha mediates cholesterol efflux in glomerular mesangial cells. Am. J. Physiol. Renal Physiol. 287, F886–F895 (2004).
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell. Metab. 7, 496–507 (2008).
Kang, K. et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell. Metab. 7, 485–495 (2008).
Kelly, K. J., Wu, P., Patterson, C. E., Temm, C. & Dominguez, J. H. LOX-1 and inflammation: a new mechanism for renal injury in obesity and diabetes. Am. J. Physiol. Renal Physiol. 294, F1136–F1145 (2008).
Letavernier, E. et al. Peroxisome proliferator-activated receptor beta/delta exerts a strong protection from ischemic acute renal failure. J. Am. Soc. Nephrol. 16, 2395–2402 (2005).
Toft, D. & Gorski, J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc. Natl Acad. Sci. USA 55, 1574–1581 (1966).
Lubahn, D. B. et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl Acad. Sci. USA 90, 11162–11166 (1993).
Barkhem, T. et al. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 54, 105–112 (1998).
Matthews, J. & Gustafsson, J. A. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol. Interv. 3, 281–292 (2003).
Neugarten, J., Acharya, A., Lei, J. & Silbiger, S. Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am. J. Physiol. Renal Physiol. 279, F309–F318 (2000).
Potier, M. et al. Estrogen-related abnormalities in glomerulosclerosis-prone mice: reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am. J. Pathol. 160, 1877–1885 (2002).
Bhat, H. K., Hacker, H. J., Bannasch, P., Thompson, E. A. & Liehr, J. G. Localization of estrogen receptors in interstitial cells of hamster kidney and in estradiol-induced renal tumors as evidence of the mesenchymal origin of this neoplasm. Cancer Res. 53, 5447–5451 (1993).
Jelinsky, S. A. et al. Global transcription profiling of estrogen activity: estrogen receptor alpha regulates gene expression in the kidney. Endocrinology 144, 701–710 (2003).
Pettersson, K., Delaunay, F. & Gustafsson, J. A. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene 19, 4970–4978 (2000).
Levin, E. R. Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 20, 477–482 (2009).
Maric, C. & Sullivan, S. Estrogens and the diabetic kidney. Gend. Med. 5 (Suppl. A), S103–S113 (2008).
Seliger, S. L., Davis, C. & Stehman-Breen, C. Gender and the progression of renal disease. Curr. Opin. Nephrol. Hypertens. 10, 219–225 (2001).
Neugarten, J., Acharya, A. & Silbiger, S. R. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J. Am. Soc. Nephrol. 11, 319–329 (2000).
Xiao, S., Gillespie, D. G., Baylis, C., Jackson, E. K. & Dubey, R. K. Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension 37, 645–650 (2001).
Catanuto, P. et al. 17 Beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 75, 1194–1201 (2009).
Holick, M. F. Vitamin D and bone health. J. Nutr. 126 (4 Suppl.), 1159S–1164S (1996).
Haussler, M. R. et al. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 66 (Suppl. 2), S98–S112 (2008).
Ordóñez-Morán, P. & Muñoz, A. Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle 8, 1675–1680 (2009).
Tan, X., Wen, X. & Liu, Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J. Am. Soc. Nephrol. 19, 1741–1752 (2008).
Kumar, R., Schaefer, J., Grande, J. P. & Roche, P. C. Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am. J. Physiol. 266, F477–F485 (1994).
Zhang, Z. et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 72, 193–201 (2007).
Wang, Y. et al. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 70, 882–891 (2006).
Zipitis, C. S. & Akobeng, A. K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch. Dis. Child. 93, 512–517 (2008).
Pittas, A. G., Lau, J., Hu, F. B. & Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 92, 2017–2029 (2007).
Levin, A. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 71, 31–38 (2007).
Teng, M. et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J. Am. Soc. Nephrol. 16, 1115–1125 (2005).
Zhang, Z. et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 73, 163–171 (2008).
Zhang, Z. et al. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc. Natl Acad. Sci. USA 105, 15896–15901 (2008).
Zhang, Y. et al. Long-term therapeutic effect of vitamin D analog doxercalciferol on diabetic nephropathy: strong synergism with AT1 receptor antagonist. Am. J. Physiol. Renal Physiol. 297, F791–F801 (2009).
Deb, D. K. et al. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-{kappa}B pathway. Am. J. Physiol. Renal Physiol. 296, F1212–F1218 (2009).
Lambers Heerspink, H. J. et al. The selective vitamin D receptor activator for albuminuria lowering (VITAL) study: study design and baseline characteristics. Am. J. Nephrol. 30, 280–286 (2009).
Katona, B. W. et al. Synthesis, characterization, and receptor interaction profiles of enantiomeric bile acids. J. Med. Chem. 50, 6048–6058 (2007).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001).
Xie, W. et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl Acad. Sci. USA 98, 3375–3380 (2001).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Gilad, L. A. & Schwartz, B. Association of estrogen receptor beta with plasma-membrane caveola components: implication in control of vitamin D receptor. J. Mol. Endocrinol. 38, 603–618 (2007).
Sladek, F. M., Zhong, W. M., Lai, E. & Darnell, J. E. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 4, 2353–2365 (1990).
Hertz, R., Magenheim, J., Berman, I. & Bar-Tana, J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature 392, 512–516 (1998).
Soutoglou, E., Katrakili, N. & Talianidis, I. Acetylation regulates transcription factor activity at multiple levels. Mol. Cell 5, 745–751 (2000).
Jiang, G., Nepomuceno, L., Yang, Q. & Sladek, F. M. Serine/threonine phosphorylation of orphan receptor hepatocyte nuclear factor 4. Arch. Biochem. Biophys. 340, 1–9 (1997).
Viollet, B., Kahn, A. & Raymondjean, M. Protein kinase A-dependent phosphorylation modulates DNA-binding activity of hepatocyte nuclear factor 4. Mol. Cell Biol. 17, 4208–4219 (1997).
Chou, W. C. et al. Mechanism of a transcriptional cross talk between transforming growth factor-beta-regulated Smad3 and Smad4 proteins and orphan nuclear receptor hepatocyte nuclear factor-4. Mol. Biol. Cell 14, 1279–1294 (2003).
Sladek, F. M. & Seidel, S. D. in Nuclear Receptors and Disease (eds Burris, T. & McCabe, E. R. B.) 309–361 (Academic Press, London, 2001).
Schrem, H., Klempnauer, J. & Borlak, J. Liver-enriched transcription factors in liver function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol. Rev. 54, 129–158 (2002).
Ryffel, G. U. Mutations in the human genes encoding the transcription factors of the hepatocyte nuclear factor (HNF)1 and HNF4 families: functional and pathological consequences. J. Mol. Endocrinol. 27, 11–29 (2001).
Mohlke, K. L. & Boehnke, M. The role of HNF4A variants in the risk of type 2 diabetes. Curr. Diab. Rep. 5, 149–156 (2005).
Du, J. et al. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J. Am. Soc. Nephrol. 18, 1437–1445 (2007).
Abramowitz, J. & Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328 (2009).
Forman, B. M. et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81, 687–693 (1995).
Seol, W., Choi, H. S. & Moore, D. D. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol. Endocrinol. 9, 72–85 (1995).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Parks, D. J. et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 284, 1365–1368 (1999).
Wang, H., Chen, J., Hollister, K., Sowers, L. C. & Forman, B. M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553 (1999).
Claudel, T. et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 109, 961–971 (2002).
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J. & Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 (2008).
Lefebvre, P., Cariou, B., Lien, F., Kuipers, F. & Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89, 147–191 (2009).
Fiorucci, S. et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127, 1497–1512 (2004).
Li, Y. T., Swales, K. E., Thomas, G. J., Warner, T. D. & Bishop-Bailey, D. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler. Thromb. Vasc. Biol. 27, 2606–2611 (2007).
Hartman, H. B. et al. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J. Lipid Res. 50, 1090–1100 (2009).
Wang, X. X. et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. Renal Physiol. 297, F1587–F1596 (2009).
Hong, C. & Tontonoz, P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 18, 461–467 (2008).
Zhang, Y. et al. Liver X receptor agonist TO-901317 upregulates SCD1 expression in renal proximal straight tubule. Am. J. Physiol. Renal Physiol. 290, F1065–F1073 (2006).
Kuipers, I. et al. Activation of liver X receptor-alpha reduces activation of the renal and cardiac renin–angiotensin–aldosterone system. Lab. Invest. doi:10.1038/labinvest.2010.7.
Giguère, V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 29, 677–696 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, X., Jiang, T. & Levi, M. Nuclear hormone receptors in diabetic nephropathy. Nat Rev Nephrol 6, 342–351 (2010). https://doi.org/10.1038/nrneph.2010.56
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.56
This article is cited by
-
Nuclear receptors in podocyte biology and glomerular disease
Nature Reviews Nephrology (2021)
-
FXR activation alleviates tacrolimus-induced post-transplant diabetes mellitus by regulating renal gluconeogenesis and glucose uptake
Journal of Translational Medicine (2019)
-
FXR expression is associated with dysregulated glucose and lipid levels in the offspring kidney induced by maternal obesity
Nutrition & Metabolism (2015)
-
Anti-Fibrosis Therapy and Diabetic Nephropathy
Current Diabetes Reports (2012)
-
Liver X receptor-activating ligands modulate renal and intestinal sodium–phosphate transporters
Kidney International (2011)