Key Points
-
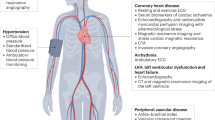
A number of available cardiac imaging tools provide information about cardiac structure and function in patients with renal disease
-
2D echocardiography is simple and non-invasive, and is a useful first-line cardiac investigative tool
-
2D echocardiography can identify structural changes associated with poor prognosis but can be prone to inaccuracy as some measurements are derived rather than actual dimensions
-
3D echocardiography is comparable to the 'gold standard' investigative tool of cardiac MRI for estimating left ventricular mass and volumes
-
Cardiac PET and single-photon emission CT provide information on myocardial perfusion in renal patients
-
Different cardiac imaging modalities may be used in combination to provide a thorough assessment of the cardiac status of renal patients
Abstract
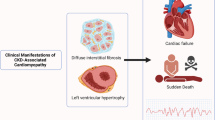
Patients with chronic kidney disease (CKD) carry a high cardiovascular risk. In this patient group, cardiac structure and function are frequently abnormal and 74% of patients with CKD stage 5 have left ventricular hypertrophy (LVH) at the initiation of renal replacement therapy. Cardiac changes, such as LVH and impaired left ventricular systolic function, have been associated with an unfavourable prognosis. Despite the prevalence of underlying cardiac abnormalities, symptoms may not manifest in many patients. Fortunately, a range of available and emerging cardiac imaging tools may assist with diagnosing and stratifying the risk and severity of heart disease in patients with CKD. Moreover, many of these techniques provide a better understanding of the pathophysiology of cardiac abnormalities in patients with renal disease. Knowledge of the currently available cardiac imaging modalities might help nephrologists to choose the most appropriate investigative tool based on individual patient circumstances. This Review describes established and emerging cardiac imaging modalities in this context, and compares their use in CKD patients with their use in the general population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
US Renal Data System. USRDS 2012 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases [online], (2012).
Foley, R. N. et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 47, 186–192 (1995).
Silberberg, J. S., Barre, P. E., Prichard, S. S. & Sniderman, A. D. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 36, 286–290 (1989).
Foley, R. N. et al. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J. Am. Soc. Nephrol. 11, 912–916 (2000).
Lang, R. M. et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463 (2005).
Park, M. et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J. Am. Soc. Nephrol. 23, 1725–1734 (2012).
Foley, R. N. et al. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J. Am. Soc. Nephrol. 5, 2024–2031 (1995).
Paoletti, E. et al. The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: a 10 year survey. Nephrol. Dial. Transplant. 19, 1829–1834 (2004).
McMahon, L. P., Roger, S. D., Levin, A. & Slimheart Investigators Group. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J. Am. Soc. Nephrol. 15, 1640–1647 (2004).
De Lima, J. J. et al. Long-term impact of renal transplantation on carotid artery properties and on ventricular hypertrophy in end-stage renal failure patients. Nephrol. Dial. Transplant. 17, 645–651 (2002).
London, G. M. et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J. Am. Soc. Nephrol. 12, 2759–2767 (2001).
Gerdts, E. Left ventricular structure in different types of chronic pressure overload. Eur. Heart J. Suppl. 10, E23–E30 (2008).
Eckardt, K. U. et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J. Am. Soc. Nephrol. 20, 2651–2660 (2009).
Philippakis, A. A. & Falk, R. H. Cardiac amyloidosis mimicking hypertrophic cardiomyopathy with obstruction: treatment with disopyramide. Circulation 125, 1821–1824 (2012).
Wang, A. Y. et al. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J. Am. Soc. Nephrol. 12, 1927–1936 (2001).
Ribeiro, S. et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol. Dial. Transplant. 13, 2037–2040 (1998).
Wang, A. Y. et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J. Am. Soc. Nephrol. 14, 159–168 (2003).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int. 76, S1–S130 (2009).
Brennan, J. M. et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin. J. Am. Soc. Nephrol. 1, 749–753 (2006).
Yamada, S. et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin. J. Am. Soc. Nephrol. 5, 1793–1798 (2010).
Zoccali, C. et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J. Am. Soc. Nephrol. 15, 1029–1037 (2004).
de Bie, M. K. et al. Prevention of sudden cardiac death: rationale and design of the Implantable Cardioverter Defibrillators in Dialysis patients (ICD2) Trial—a prospective pilot study. Curr. Med. Res. Opin. 24, 2151–2157 (2008).
Zotz, R. J., Genth, S., Waaler, A., Erbel, R. & Meyer, J. Left ventricular volume determination using Albunex. J. Am. Soc. Echocardiogr. 9, 1–8 (1996).
de Bie, M. K. et al. Left ventricular diastolic dysfunction in dialysis patients assessed by novel speckle tracking strain rate analysis: prevalence and determinants. Int. J. Nephrol. 2012, 963504 (2012).
Farshid, A., Pathak, R., Shadbolt, B., Arnolda, L. & Talaulikar, G. Diastolic function is a strong predictor of mortality in patients with chronic kidney disease. BMC Nephrol. 14, 280 (2013).
Tripepi, G. et al. Left atrial volume in end-stage renal disease: a prospective cohort study. J. Hypertens. 24, 1173–1180 (2006).
Tripepi, G. et al. Left atrial volume monitoring and cardiovascular risk in patients with end-stage renal disease: a prospective cohort study. J. Am. Soc. Nephrol. 18, 1316–1322 (2007).
Assa, S. et al. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin. J. Am. Soc. Nephrol. 7, 1615–1623 (2012).
Burton, J. O., Jefferies, H. J., Selby, N. M. & McIntyre, C. W. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 4, 914–920 (2009).
Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC) et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur. Heart J. 30, 2769–2812 (2009).
Wizemann, V. & Timio, M. Dialysis schedule-related fluid state and cardiovascular effects. Nephrol. Dial. Transplant. 13 (Suppl. 6), 91–93 (1998).
Rutsky, E. A. Treatment of uremic pericarditis and pericardial effusion. Am. J. Kidney Dis. 10, 2–8 (1987).
Jenkins, C., Bricknell, K., Hanekom, L. & Marwick, T. H. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 44, 878–886 (2004).
Mor-Avi, V. et al. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: comparison with magnetic resonance imaging. Circulation 110, 1814–1818 (2004).
Krenning, B. J. et al. Three-dimensional echocardiographic analysis of left ventricular function during hemodialysis. Nephron Clin. Pract. 107, c43–c49 (2007).
Chiu, D. Y., Green, D., Abidin, N., Sinha, S. & Kalra, P. A. Echocardiography in hemodialysis patients: uses and challenges. Am. J. Kidney Dis. 64, 804–816 (2014).
Nigri, M. et al. Contrast-enhanced magnetic resonance imaging identifies focal regions of intramyocardial fibrosis in patients with severe aortic valve disease: correlation with quantitative histopathology. Am. Heart J. 157, 361–368 (2009).
Syed, I. S. et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc. Imaging 3, 155–164 (2010).
Kim, R. J. et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 343, 1445–1453 (2000).
Pazos, P. et al. Diagnostic value of cardiac magnetic resonance for the differential diagnosis of thrombus vs tumor [poster presentation]. J. Cardiovasc. Magn. Reson. 15 (Suppl. 1), P103 (2013).
Bogaert, J. & Francone, M. Cardiovascular magnetic resonance in pericardial diseases. J. Cardiovasc. Magn. Reson. 11, 14 (2009).
Rumberger, J. A., Sheedy, P. F., Breen, J. F. & Schwartz, R. S. Electron beam computed tomographic coronary calcium score cutpoints and severity of associated angiographic lumen stenosis. J. Am. Coll. Cardiol. 29, 1542–1548 (1997).
Bellasi, A. & Raggi, P. Techniques and technologies to assess vascular calcification. Semin. Dial. 20, 129–133 (2007).
Braun, J. et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am. J. Kidney Dis. 27, 394–401 (1996).
Matsuoka, M. et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin. Exp. Nephrol. 8, 54–58 (2004).
Shantouf, R. S. et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am. J. Nephrol. 31, 419–425 (2010).
Sharples, E. J. et al. Coronary artery calcification measured with electron-beam computerized tomography correlates poorly with coronary artery angiography in dialysis patients. Am. J. Kidney Dis. 43, 313–319 (2004).
O'Rourke, R. A. et al. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 102, 126–140 (2000).
Rufino, M. et al. Heart valve calcification and calcium x phosphorus product in hemodialysis patients: analysis of optimum values for its prevention. Kidney Int. 63, (Suppl. 85), S115–S118 (2003).
Gulel, O. et al. Evidence of left ventricular systolic and diastolic dysfunction by color tissue Doppler imaging despite normal ejection fraction in patients on chronic hemodialysis program. Echocardiography 25, 569–574 (2008).
Sharma, R. et al. Reduced longitudinal myocardial tissue velocities and myocardial deformation with strain rate imaging in patients with end stage renal disease and apparent normal left ventricular ejection fraction [abstract 954]. Eur. J. Echocardiogr. 7 (Suppl. 1), S163–S164 (2006).
Fijalkowski, M. et al. Effect of preload reduction by hemodialysis on myocardial ultrasonic characterization, left atrial volume, and Doppler tissue imaging in patients with end-stage renal disease. J. Am. Soc. Echocardiogr. 19, 1359–1364 (2006).
le, E. H. et al. Preload dependence of new Doppler techniques limits their utility for left ventricular diastolic function assessment in hemodialysis patients. J. Am. Soc. Nephrol. 14, 1858–1862 (2003).
Hung, K. C. et al. Evaluating preload dependence of a novel Doppler application in assessment of left ventricular diastolic function during hemodialysis. Am. J. Kidney Dis. 43, 1040–1046 (2004).
Amundsen, B. H. et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 47, 789–793 (2006).
Yan, P. et al. 2D-Speckle tracking echocardiography contributes to early identification of impaired left ventricular myocardial function in patients with chronic kidney disease. Nephron Clin. Pract. 118, c232–c240 (2011).
Liu, Y. W. et al. Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin. J. Am. Soc. Nephrol. 8, 1564–1574 (2013).
Aoki, J. et al. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 67, 333–340 (2005).
Liu, Y. W. et al. Application of speckle-tracking echocardiography in detecting coronary artery disease in patients with maintenance hemodialysis. Blood Purif. 32, 38–42 (2011).
Choi, J. O. et al. Effect of preload on left ventricular longitudinal strain by 2D speckle tracking. Echocardiography 25, 873–879 (2008).
Jia, C. et al. Comparison of 2-D speckle tracking and tissue Doppler imaging in an isolated rabbit heart model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 57, 2491–2502 (2010).
Ibrahim, el-S. H. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques—pulse sequences, analysis algorithms, and applications. J. Cardiovasc. Magn. Reson. 13, 36 (2011).
Edwards, N. C. et al. Impaired circumferential and longitudinal myocardial deformation in early stage chronic kidney disease: the earliest features of uremic cardiomyopathy [poster presentation]. J. Cardiovasc. Magn. Reson. 15 (Suppl. 1), P153 (2013).
Herzog, C. A. et al. Dobutamine stress echocardiography for the detection of significant coronary artery disease in renal transplant candidates. Am. J. Kidney Dis. 33, 1080–1090 (1999).
Karagiannis, S. E. et al. Prognostic significance of renal function in patients undergoing dobutamine stress echocardiography. Nephrol. Dial. Transplant. 23, 601–607 (2008).
Rakhit, D. J., Armstrong, K. A., Beller, E., Isbel, N. M. & Marwick, T. H. Risk stratification of patients with chronic kidney disease: results of screening strategies incorporating clinical risk scoring and dobutamine stress echocardiography. Am. Heart J. 152, 363–370 (2006).
Bergeron, S. et al. Prognostic value of dobutamine stress echocardiography in patients with chronic kidney disease. Am. Heart J. 153, 385–391 (2007).
Sharma, R. et al. Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol. Dial. Transplant. 20, 2207–2214 (2005).
Schmidt, A., Stefenelli, T., Schuster, E. & Mayer, G. Informational contribution of noninvasive screening tests for coronary artery disease in patients on chronic renal replacement therapy. Am. J. Kidney Dis. 37, 56–63 (2001).
Cordeiro, A. C. et al. Reliability of electrocardiographic surrogates of left ventricular mass in patients with chronic kidney disease. J. Hypertens. 32, 439–445 (2014).
Kharabsheh, S. M., Al-Sugair, A., Al-Buraiki, J. & Al-Farhan, J. Overview of exercise stress testing. Ann. Saudi Med. 26, 1–6 (2006).
Atkinson, P. et al. Predictive value of myocardial and coronary imaging in the long-term outcome of potential renal transplant recipients. Int. J. Cardiol. 146, 191–196 (2011).
Wong, C. F., Little, M. A., Vinjamuri, S., Hammad, A. & Harper, J. M. Technetium myocardial perfusion scanning in prerenal transplant evaluation in the United Kingdom. Transplant. Proc. 40, 1324–1328 (2008).
De Vriese, A. S. et al. Comparison of the prognostic value of dipyridamole and dobutamine myocardial perfusion scintigraphy in hemodialysis patients. Kidney Int. 76, 428–436 (2009).
Lentine, K. L. et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 60, 434–480 (2012).
Ragosta, M. et al. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am. Heart J. 147, 1017–1023 (2004).
Lubberink, M. et al. Low-dose quantitative myocardial blood flow imaging using 15O-water and PET without attenuation correction. J. Nucl. Med. 51, 575–580 (2010).
Knuuti, J., Kajander, S., Mäki, M. & Ukkonen, H. Quantification of myocardial blood flow will reform the detection of CAD. J. Nucl. Cardiol. 16, 497–506 (2009).
Di Carli, M. F. & Murthy, V. L. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics 31, 1239–1254 (2011).
Stewart, R. E. et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am. J. Cardiol. 67, 1303–1310 (1991).
Bateman, T. M. et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J. Nucl. Cardiol. 13, 24–33 (2006).
McIntyre, C. W. et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin. J. Am. Soc. Nephrol. 3, 19–26 (2008).
Grover-McKay, M. et al. Detection of coronary artery disease with positron emission tomography and rubidium 82. Am. Heart J. 123, 646–652 (1992).
Tamaki, N. et al. Value and limitation of stress thallium-201 single photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J. Nucl. Med. 29, 1181–1188 (1988).
Marwick, T. H., Nemec, J. J., Stewart, W. J. & Salcedo, E. E. Diagnosis of coronary artery disease using exercise echocardiography and positron emission tomography: comparison and analysis of discrepant results. J. Am. Soc. Echocardiogr. 5, 231–238 (1992).
Sampson, U. K., Dorbala, S., Limaye, A., Kwong, R. & Di Carli, M. F. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J. Am. Coll. Cardiol. 49, 1052–1058 (2007).
Pisaniello, A. D. et al. Dobutamine stress cardiac MRI reliably predicts significant coronary disease in renal transplant candidates [poster presentation]. J. Cardiovasc. Magn. Reson. 16 (Suppl. 1), P181 (2014).
Grobner, T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 21, 1104–1108 (2006).
Mowatt, G. et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 94, 1386–1393 (2008).
de Graaf, F. R. et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography in the non-invasive evaluation of significant coronary artery disease. Eur. Heart J. 31, 1908–1915 (2010).
Cannata-Andía, J. B., Rodríguez-García, M., Carrillo-López, N., Naves-Díaz, M. & Díaz-López, B. Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J. Am. Soc. Nephrol. 17 (12 Suppl. 3), S267–S273 (2006).
de Bie, M. K. et al. CT coronary angiography is feasible for the assessment of coronary artery disease in chronic dialysis patients, despite high average calcium scores. PLoS ONE 8, e67936 (2013).
Mao, J. et al. Coronary computed tomography angiography in dialysis patients undergoing pre-renal transplantation cardiac risk stratification. Cardiol. J. 17, 349–361 (2010).
Chrysochou, C. et al. BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol. Dial. Transplant. 27, 1013–1019 (2012).
Arnold, J. R. et al. Myocardial oxygenation in coronary artery disease: insights from blood oxygen level-dependent magnetic resonance imaging at 3 tesla. J. Am. Coll. Cardiol. 59, 1954–1964 (2012).
Xiao, W., Xu, J., Wang, Q., Xu, Y. & Zhang, M. Functional evaluation of transplanted kidneys in normal function and acute rejection using BOLD MR imaging. Eur. J. Radiol. 81, 838–845 (2012).
Karamitsos, T. D. et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ. Cardiovasc. Imaging 3, 32–40 (2010).
Kajander, S. et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 122, 603–613 (2010).
Dudley, C. & Harden, P. Module 4: Assessment for renal transplantation. UK Renal Association Clinical Practice Guidelines [online], (2008).
Chertow, G. M., Burke, S. K., Raggi, P. & Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 62, 245–252 (2002).
Raggi, P. et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J. Bone Miner. Res. 20, 764–772 (2005).
Block, G. A., Raggi, P., Bellasi, A., Kooienga, L. & Spiegel, D. M. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 71, 438–441 (2007).
Qunibi, W. et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am. J. Kidney Dis. 51, 952–965 (2008).
Raggi, P. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol. Dial. Transplant. 26, 1327–1339 (2011).
Barreto, D. V. et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRiC study. Nephron Clin. Pract. 110, c273–c283 (2008).
Russo, D. et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 72, 1255–1261 (2007).
Delos Santos, R. B., Gmurczyk, A., Obhrai, J. S. & Watnick, S. G. Cardiac evaluation prior to kidney transplantation. Semin. Dial. 23, 324–329 (2010).
Acknowledgements
D.Y.Y.C., D.G., N.A. and P.A.K. are in receipt of a Kidney Research UK project grant to investigate sudden cardiac death in dialysis patients; however, Kidney Research UK had no role in writing, reviewing or authorizing this manuscript and this article does not include data pertaining to the aforementioned study.
Author information
Authors and Affiliations
Contributions
D.Y.Y.C researched the material for this article. D.Y.Y.C., D.G., N.A., S.S. and P.A.K. contributed to discussion of content, writing and editing of the manuscript. P.A.K. managed the overall writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.Y.Y.C. has received honoraria for travel expenses from Amgen Bursary and Takeda to attend conferences. S.S. has received honoraria for speaking from Amgen, Fresenius and Shire, and receives an Amgen educational grant for the UK Calciphylaxis Study. P.A.K. has received honoraria for speaker meetings, advisory boards and other consultancy work from Amgen, Boehringer Ingelheim, Fresenius, MSD, Novartis, Otsuka, Pfizer, Pharmacosmos, Reata, Sanofi, Shire, Takeda and Vifor. His department has received educational grants from Amgen, Sanofi and Shire. D.G. and N.A. declare no competing interests.
Rights and permissions
About this article
Cite this article
Chiu, D., Green, D., Abidin, N. et al. Cardiac imaging in patients with chronic kidney disease. Nat Rev Nephrol 11, 207–220 (2015). https://doi.org/10.1038/nrneph.2014.243
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.243
This article is cited by
-
Assessment and management of heart failure in patients with chronic kidney disease
Heart Failure Reviews (2023)
-
Implications of uremic cardiomyopathy for the practicing clinician: an educational review
Heart Failure Reviews (2023)
-
Assessment of cardiac structure and function in kidney failure: understanding echocardiography and magnetic resonance imaging for the nephrologist
International Urology and Nephrology (2021)
-
Relative overhydration is independently associated with left ventricular hypertrophy in dialysis naïve patients with stage 5 chronic kidney disease
Scientific Reports (2020)
-
Epicardial adipose tissue in patients with chronic kidney disease: a meta-analysis study and trial sequential analysis
International Urology and Nephrology (2020)