Abstract
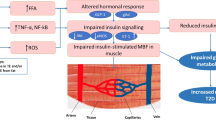
The increasing incidence of obesity and the metabolic syndrome over the past two decades has coincided with a marked increase in total fructose intake. Fructose—unlike other sugars—causes serum uric acid levels to rise rapidly. We recently reported that uric acid reduces levels of endothelial nitric oxide (NO), a key mediator of insulin action. NO increases blood flow to skeletal muscle and enhances glucose uptake. Animals deficient in endothelial NO develop insulin resistance and other features of the metabolic syndrome. As such, we propose that the epidemic of the metabolic syndrome is due in part to fructose-induced hyperuricemia that reduces endothelial NO levels and induces insulin resistance. Consistent with this hypothesis is the observation that changes in mean uric acid levels correlate with the increasing prevalence of metabolic syndrome in the US and developing countries. In addition, we observed that a serum uric acid level above 5.5 mg/dl independently predicted the development of hyperinsulinemia at both 6 and 12 months in nondiabetic patients with first-time myocardial infarction. Fructose-induced hyperuricemia results in endothelial dysfunction and insulin resistance, and might be a novel causal mechanism of the metabolic syndrome. Studies in humans should be performed to address whether lowering uric acid levels will help to prevent this condition.
Key Points
-
Increased ingestion of fructose in processed foodstuffs has correlated with development of the obesity epidemic
-
Hypothesis: fructose-mediated elevation of serum uric acid levels has a role in development of the metabolic syndrome
-
By the proposed hypothesis, transient hyperuricemia would exert its effect by limiting bioavailability of endothelial nitric oxide, leading to insulin resistance and hypertension
-
Low-fructose diets or allopurinol-mediated lowering of serum uric acid levels might prevent or successfully treat early stage metabolic syndrome
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hedley AA et al. (2004) Prevalence of overweight and obesity among children, adolescents, and adults, 1999–2002. JAMA 2: 2847–2850
Weiss R et al. (2004) Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374
Block G (2004) Foods contributing to energy intake in the U.S.: data from NHANES III and NHANES 1999–2000: J Food Comp Analysis 17: 439–447
Johnson RJ et al. (2005) Uric acid, evolution, and primitive cultures. Sem Nephrol 25: 3–8
Yusuf S et al. (2001) Global burden of cardiovascular diseases. Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104: 2746–2753
Serdula MK et al. (1999) Prevalence of attempting weight loss and strategies for controlling weight. JAMA 282: 1353–1358
Foster GD et al. (2003) A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 348: 2082–2089
United States Department of Agriculture Economic Research Service report. [http://www.ers.usda.gov/data/foodconsumption/spreadsheets/sweets.xls]
US Census Bureau (2003) Health and Nutrition. No. 214 Per Capita Consumption of Major Food Commodities: 1980 to 2001. In: Statistical Abstract of the United States: 2003, 143. Washington DC: US Census Bureau
Bray GA et al. (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79: 537–543
Ludwig DS et al. (2001) Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 357: 505–508
Schulze MB et al. (2004) Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 292: 927–934
Dennison BA et al. (1997) Excess fruit juice consumption by preschool-aged children is associated with short stature and obesity. Pediatr 99: 15–22
Hallfrisch J (1990) Metabolic effects of dietary fructose. FASEB J 4: 2652–2660
Lingelbach LB and McDonald RB (2000) Description of the long-term lipogenic effects of dietary carbohydrates in male Fischer 344 rats. J Nutr 130: 3077–3084
Teff KL et al. (2004) Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 89: 2963–2972
Zammit VA et al. (2001) Insulin stimulation of hepatic triacylglycerol secretion and the etiology of insulin resistance. J Nutr 131: 2074–2077
Perheentupa J and Raivio K (1967) Fructose induced hyperuricemia. Lancet 2: 528–535
Emmerson BT (1974) Effect of oral fructose on urate production. Ann Rheum Dis 33: 276–280
Stirpe F et al. (1970) Fructose-induced hyperuricemia. Lancet 2: 1310–1311
Fox IH and Kelley WN (1972) Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 21: 713–721
Smith CM et al. (1977) Fructose-induced adenine nucleotide catabolism in isolated rat hepatocyte. Can J Biochem 55: 1237–1240
Khosla UM et al. (2005) Hyperuricemia reversibly inhibits endothelial dysfunction in rats. Kidney Int 67: 1739–1742
Kang DH et al. (2003) Uric acid induced C-reactive protein (CRP) expression via upregulation of angiotensin type 1 receptors (AT1) in vascular endothelial cells and smooth muscle cells [abstract #136]. J Am Soc Nephrol 14
Kanabrocki EL et al. (2000) Circadian relationship of serum uric acid and nitric oxide. JAMA 283: 2240–2241
Butler R et al. (2000) Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35: 746–751
Doehner W et al. (2002) Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 105: 2619–2624
Farquharson CA et al. (2002) Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 106: 221–226
Cardillo C et al. (1997) Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension 30: 57–63
Guthikonda S et al. (2003) Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107: 416–421
Scherrer U et al. (1994) Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest 94: 2511–2515
Cook S et al. (2003) Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly 133: 360–363
Roy D et al. (1998) Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol 274: E692–E699
Wexler BC and Greenberg BP (1977) Effect of increased serum urate levels on virgin rats with no arteriosclerosis versus breeder rats with preexistent arteriosclerosis. Metabolism 26: 1309–1320
Wexler BC (1982) Allantoxanamide-induced myocardial necrosis in Sprague-Dawley vs. Spontaneously Hypertensive Rats. Proc Soc Exp Biol Med 170: 476–485
Johnson RJ et al. (2005) The resurrection of uric acid as a causal factor in hypertension. Hypertension 45: 18–20
Masuo K et al. (2003) Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42: 474–480
Nakanishi N et al. (2003) Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type 2 diabetes in Japanese male office workers. Eur J Epidemiol 18: 523–530
Sundström J et al. (2005) Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 45: 28–33
Vuorinen-Markkola H and Yki-Järvinen H (1994) Hyperuricemia and insulin resistance. J Clin Endo Metab 78: 25–29
Costa A et al. (2002) Uric acid concentration in subjects at risk of type 2 diabetes mellitus: Relationship to components of the metabolic syndrome. Metabolism 51: 372–375
Berkowitz D (1966) Gout, hyperlipidemia, and diabetes interrelationships. JAMA 197: 117–120
Thadhani R et al. (2004) Insulin resistance and alterations in angiogenesis. Additive insults that may lead to preeclampsia. Hypertension 43: 988–992
Pascual E (1994) Hyperuricemia and gout. Curr Opin Rheumatol 6: 454–458
Lindholm LH et al. (2003) Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid profile in a North of Sweden Efficacy Evaluation (ALPIN study). J Hypertens 21: 1563–1574
Deedwania PC (2003) Mechanisms of endothelial dysfunction in the metabolic syndrome. Curr Diab Rep 3: 289–292
Fishberg AM (1924) The interpretation of increased blood uric acid in hypertension. Arch Int Med 34: 503–507
Hall AP et al. (1967) Epidemiology of gout and hyperuricemia. Am J Med 42: 27–37
Mikkelsen WM et al. (1965) The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia. Tecumseh, Michigan. 1959–1960. Am J Med 39: 242–251
Glynn RJ et al. (1983) Trends in serum uric acid levels. 1961–1980. Arthritis Rheum 26: 87–93
Freedman DS et al. (1995) Relation of serum uric acid to mortality and ischemic heart disease. Am J Epidemiol 141: 637–644
Gresser U et al. (1990) Uric acid levels in Southern Germany in 1989. A comparison with studies from 1962, 1971, and 1984. Klin Wochenschr 68: 1222–1228
Robinson SC and Brucer M (1939) Range of normal blood pressure. A statistical and clinical study of 11,383 persons. Arch Int Med 64: 409–444
Arromedee E et al. (2002) Epidemiology of gout: Is the incidence rising? J Rheumatol 29: 2403–2406
Lennane GAQ et al. (1960) Gout in the Maori. Ann Rheum Dis 19: 120–125
Conen D et al. (2004) Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health 4: 1–9
Kagan A et al. (1974) Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii, and California. Demographic, physical, dietary and biochemical characteristics. J Chron Dis 27: 345–364
Choi HK et al. (2004) Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 350: 1093–1103
Johnson RJ and Rideout BA (2004) Uric acid and diet: insights into the epidemic of cardiovascular disease. N Engl J Med 350: 1071–1073
Tuomilehto J et al. (1988) Plasma uric acid level and its association with diabetes mellitus and some biological parameters in a biracial population of Fiji. Am J Epidemiol 127: 321–336
Bo S et al. (2001) Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31: 318–321
Sánchez-Lozada LG et al. (2005) Mild hyperuricemia induces severe cortical vasoconstriction and perpetuates glomerular hypertension in normal rats and in experimental chronic renal failure. Kidney Int 67: 237–234
Kang DH et al. (2002) A role for uric acid in renal progression. J Am Soc Nephrol 13: 2888–2897
Beckman JA et al. (2002) Inhibition of protein kinase C β prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res 90: 107–111
Hogan M et al. (1992) Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest 90: 1110–1115
Mazzali M et al. (2001) Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106
Shinozaki K et al. (2004) Evidence for a causal role of the renin angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension 43: 255–262
Pollock DM et al. (1993) Angiotensin blockade reverses hypertension during long-term nitric oxide blockade. Hypertension 21: 660–666
Yusuf S et al. (2001) HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 286: 1882–1885
Osei K et al. (1987) Metabolic effects of fructose as a natural sweetener in the physiological meals of ambulatory obese patients with type 2 diabetes. Am J Med 83: 249–255
Galvan AQ et al. (1995) Effect of insulin on uric acid excretion in humans. Am J Physiol 268: E1–E5
Prior IAM et al. (1966) Hyperuricemia, gout and diabetic abnormality in Polynesian people. Lancet 1: 333–338
Fisher ND et al. (2003) Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286
Waring WS et al. (2004) Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart 90: 155–159
Osler W (1893) Gout. In: The Principles and Practice of Medicine, edn 2, 287–295. New York: Appleton
Dessein PH et al. (2000) Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout; a pilot study. Ann Rheum Dis 59: 539–543
Acknowledgements
This study was supported by NIH grants DK-52121, HL-68607, a George O'Brien Center grant (DK-P50-DK064233) and a pilot grant from the Juvenile Diabetes Foundation. We thank Amy Buhler for help in obtaining some of the older references, and Edward R Block, Daniel I Feig, Olena Glushakova, Jaime Herrera-Acosta, Hanbo Hu, Xiaosen Ouyang and Sergey Zharikov for assistance with experimental studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Johnson and Dr Nakagawa have submitted a patent application on lowering uric acid as a means for preventing or treating the metabolic syndrome. Dr Johnson is also a consultant for TAP Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Nakagawa, T., Tuttle, K., Short, R. et al. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Rev Nephrol 1, 80–86 (2005). https://doi.org/10.1038/ncpneph0019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0019
This article is cited by
-
Hyperuricemia: An Intriguing Connection to Metabolic Syndrome, Diabetes, Kidney Disease, and Hypertension
Current Hypertension Reports (2024)
-
The predictive role of serum uric acid levels before pregnancy in the development of gestational diabetes mellitus
Diabetology International (2024)
-
Responses to Hypoxia: How Fructose Metabolism and Hypoxia-Inducible Factor-1a Pathways Converge in Health and Disease
Current Nutrition Reports (2023)
-
The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases
Nature Reviews Endocrinology (2022)
-
Therapeutic RNA-silencing oligonucleotides in metabolic diseases
Nature Reviews Drug Discovery (2022)