Key Points
-
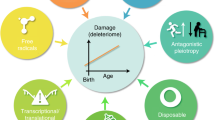
Ageing affects neurons as much as it does cells in other organ systems. During ageing, neurons experience increased amounts of oxidative stress, perturbed energy homeostasis, accumulation of damaged proteins and lesions in their DNA.
-
Studies of mutations in the genes that cause Huntington's disease or early-onset forms of Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis have provided evidence that the defective genes exacerbate age-related processes in neurons, including aggregation of pathogenic proteins, oxidative stress and perturbed cellular ion homeostasis.
-
The physical and molecular characteristics of neurons, their functional properties and their location in neural circuits are all likely to influence their fate during ageing. Examples include differences in relative vulnerabilities to excitotoxicity, metabolic stress and neurotoxins.
-
Neurons have an array of mechanisms that might protect them against the adversities of ageing and neurodegenerative disorders, such as antioxidant defences, protein chaperones that prevent protein aggregation and neurotrophic factors that stabilize ion homeostasis and mitochondrial function.
-
Synapses are regions of neurons that are particularly vulnerable to ageing and neurodegenerative disorders. They are prone to excitotoxic damage, as well as damage by pathogenic proteins such as amyloid-β peptide and mutant huntingtin.
-
Data from studies of animal models and human populations suggest that factors that promote healthy ageing, such as regular exercise and reduced energy diets, might also protect the nervous system against neurodegenerative disorders.
Abstract
Everyone ages, but only some will develop a neurodegenerative disorder in the process. Disease might occur when cells fail to respond adaptively to age-related increases in oxidative, metabolic and ionic stress, thereby resulting in the accumulation of damaged proteins, DNA and membranes. Determinants of neuronal vulnerability might include cell size and location, metabolism of disease-specific proteins and a repertoire of signal transduction pathways and stress resistance mechanisms. Emerging evidence on protein interaction networks that monitor and respond to the normal ageing process suggests that successful neural ageing is possible for most people, but also cautions that cures for neurodegenerative disorders are unlikely in the near future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hofer, S. M., Berg, S. & Era, P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychol. Aging 18, 285–305 (2003).
Mattson, M. P. Pathways towards and away from Alzheimer's disease. Nature 430, 631–639 (2004). This review integrates information on the molecular pathogenesis of AD and efforts to prevent and treat this disorder.
Cookson, M. R. The biochemistry of Parkinson's disease. Annu. Rev. Biochem. 74, 29–52 (2005).
Moore, D. J., West, A. B., Dawson, V. L. & Dawson, T. M. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 27, 57–87 (2005). Reviews the involvement of mitochondrial dysfunction and perturbed ubiquitin-mediated proteolysis in PD.
Sieradzan, K. A. & Mann, D. M. The selective vulnerability of nerve cells in Huntington's disease. Neuropathol. Appl. Neurobiol. 27, 1–21 (2001).
Cleveland, D. W. & Rothstein, J. D. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nature Rev. Neurosci. 2, 806–819 (2001).
Serrano, F. & Klann, E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 3, 431–443 (2004).
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R. & Crichton, R. R. Iron, brain ageing and neurodegenerative disorders. Nature Rev. Neurosci. 5, 863–873 (2004).
Ames, B. N. Delaying the mitochondrial decay of aging. Ann. NY Acad. Sci. 1019, 406–411 (2004).
Gray, D. A., Tsirigotis, M. & Woulfe, J. Ubiquitin, proteasomes, and the aging brain. Sci. Aging Knowledge Environ. RE6 27 Aug 2003.
Trojanowski, J. Q. & Mattson, M. P. Overview of protein aggregation in single, double, and triple neurodegenerative brain amyloidoses. Neuromolecular Med. 4, 1–6 (2003).
Kyng, K. J. & Bohr, V. A. Gene expression and DNA repair in progeroid syndromes and human aging. Ageing Res. Rev. 4, 579–602 (2005).
Lu, T. et al. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 (2004).
Butler, R. N. et al. Longevity genes: from primitive organisms to humans. J. Gerontol. A Biol. Sci. Med. Sci. 58, 581–584 (2003).
Wolkow, C. A. Life span: getting the signal from the nervous system. Trends Neurosci. 25, 212–216 (2002). Describes how insulin-like signalling in the nervous system might regulate ageing and determine lifespan.
Mattson, M. P. Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res. Rev. 2, 329–342 (2003).
Yehuda, S., Rabinovitz, S., Carasso, R. L. & Mostofsky, D. I. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol. Aging 23, 843–853 (2002).
Floyd, R. A. & Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 23, 795–807 (2002).
Jenner, P. Oxidative stress in Parkinson's disease. Ann. Neurol. 53, S26–S36 (2003).
Pedersen, W. A. et al. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 44, 819–824 (1998).
Rakhit, R. et al. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J. Biol. Chem. 279, 15499–15504 (2004).
Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T. & Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004). Describes the degradation of wild-type α-synuclein by chaperone-mediated autophagy, and the impairment of this process by mutant forms of the protein.
Grondin, R. et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain 125, 2191–2201 (2002).
Zuccato, C. et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nature Genet. 35, 76–83 (2003).
Weindruch, R. & Sohal, R. S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 337, 986–994 (1997).
Heilbronn, L. K. & Ravussin, E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 78, 361–369 (2003).
Mattison, J. A., Lane, M. A., Roth, G. S. & Ingram, D. K. Calorie restriction in rhesus monkeys. Exp. Gerontol. 38, 35–46 (2003).
Fontana, L., Meyer, T. E., Klein, S. & Holloszy, J. O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl Acad. Sci. USA 101, 6659–6663 (2004).
Mattson, M. P. Energy intake, meal frequency, and health: a neurobiological perspective. Annu. Rev. Nutr. 25, 237–260 (2005).
Patel, N. V. et al. Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models. Neurobiol. Aging 26, 995–1000 (2005).
Maswood, N. et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc. Natl Acad. Sci. USA 101, 18171–18176 (2004).
Duan, W. et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl Acad. Sci. USA 100, 2911–2916 (2003).
Yu, Z. F. & Mattson, M. P. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 57, 830–839 (1999).
Mattson, M. P., Maudsley, S. & Martin, B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 27, 589–594 (2004).
Barja, G. Free radicals and aging. Trends Neurosci. 27, 595–600 (2004).
Cotman, C. W. & Berchtold, N. C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301 (2002).
Spires, T. L. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J. Neurosci. 24, 2270–2276 (2004).
Liu, D. et al. Mitochondrial UCP4 induces a glycolytic shift in energy metabolism and increases the resistance of neurons to oxidative stress. Neuromolecular Med. (in the press).
Parker, J. A. et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genet. 37, 349–350 (2005).
Morrison, B. M., Hof, P. R. & Morrison, J. H. Determinants of neuronal vulnerability in neurodegenerative diseases. Ann. Neurol. 44, S32–S44 (1998).
Smith, D. E., Saji, M., Joh, T. H., Reis, D. J. & Pickel, V. M. Ibotenic acid-induced lesions of striatal target and projection neurons: ultrastructural manifestations in dopaminergic and non-dopaminergic neurons and in glia. Histol. Histopathol. 2, 251–263 (1987).
Mandelkow, E. M., Stamer, K., Vogel, R., Thies, E. & Mandelkow, E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging 24, 1079–1085 (2003).
Bayatti, N. & Behl, C. The neuroprotective actions of corticotropin releasing hormone. Ageing Res. Rev. 4, 258–270 (2005).
Ishunina, T. A. & Swaab, D. F. Neurohypophyseal peptides in aging and Alzheimer's disease. Ageing Res. Rev. 1, 537–558 (2002).
Braak, H. & Braak, E. Evolution of neuronal changes in the course of Alzheimer's disease. J. Neural Transm. Suppl. 53, 127–140 (1998).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Mills, K. R. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain 126, 2558–2566 (2003).
Yuan, J. & Yankner, B. A. Apoptosis in the nervous system. Nature 407, 802–809 (2000). Reviews evidence that supports a role for apoptosis in the pathogenesis of neurodegenerative disorders.
Mattson, M. P. Apoptosis in neurodegenerative disorders. Nature Rev. Mol. Cell Biol. 1, 120–129 (2000).
Miller, F. D., Pozniak, C. D. & Walsh, G. S. Neuronal life and death: an essential role for the p53 family. Cell Death Differ. 7, 880–888 (2000).
Tanaka, Y. et al. Inducible expression of mutant α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 10, 919–926 (2001).
Akhtar, R. S., Ness, J. M. & Roth, K. A. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim. Biophys. Acta 1644, 189–203 (2004).
Cheung, E. C. et al. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J. Neurosci. 25, 1324–1334 (2005).
Wang, K. K. Calpain and caspase: can you tell the difference? Trends Neurosci. 23, 20–26 (2000).
Stefanis, L. Caspase-dependent and -independent neuronal death: two distinct pathways to neuronal injury. Neuroscientist 11, 50–62 (2005).
Chan, S. L. et al. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol. Dis. 11, 2–19 (2002).
Brown, D. & Tatton, N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann. Neurol. 53 (Suppl. 3), S61–S70 (2003).
Crocker, S. J. et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J. Neurosci. 23, 4081–4091 (2003).
Gafni, J. & Ellerby, L. M. Calpain activation in Huntington's disease. J. Neurosci. 22, 4842–4849 (2002).
Friedlander, R. M. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 348, 1365–1375 (2003).
Mattson, M. P. & Kroemer, G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol. Med. 9, 196–205 (2003).
Glazner, G. W. et al. Endoplasmic reticulum D-myo-inositol 1,4,5-trisphosphate-sensitive stores regulate nuclear factor-κB binding activity in a calcium-independent manner. J. Biol. Chem. 276, 22461–22467 (2001).
Milhavet, O. et al. Involvement of Gadd153 in the pathogenic action of presenilin-1 mutations. J. Neurochem. 83, 673–681 (2002).
Boehning, D. et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nature Cell Biol. 5, 1051–1061 (2003).
Culmsee, C. & Mattson, M. P. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 331, 761–777 (2005).
Kruman, I. et al. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J. Neurosci. 17, 5089–5100 (1997).
Bruckner, S. R., Perry, G. & Estus, S. 4-hydroxynonenal contributes to NGF withdrawal-induced neuronal apoptosis. J. Neurochem. 85, 999–1005 (2003).
Zhou, H., Li, X. M., Meinkoth, J. & Pittman, R. N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 151, 483–494 (2000).
Cutler, R. G. et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl Acad. Sci. USA 101, 2070–2075 (2004). Links age-related alterations in the metabolism of membrane lipids to oxidative stress and neuronal degeneration in AD.
Haughey, N. J. et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. 55, 257–267 (2004).
Cutler, R. G. et al. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann. Neurol. 52, 448–457 (2002).
White, L. D. & Barone, S. Jr Qualitative and quantitative estimates of apoptosis from birth to senescence in the rat brain. Cell Death Differ. 8, 345–356 (2001).
Hiona, A. & Leeuwenburgh, C. Life-long caloric restriction counteracts apoptotic effects of aging in the brain and bolsters the action of apoptosis inhibitors. Neurobiol. Aging. 26 Sep 2006 (doi: 10.1016/j.neurobiolaging.2005.08.006).
Kempermann, G., Kuhn, H. G. & Gage, F. H. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18, 3206–3212 (1998). Evidence that age-related declines in hippocampal neurogenesis can be counteracted by cognitive stimulation.
Sun, W. et al. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J. Neurosci. 24, 11205–11213 (2004).
Haughey, N. J. et al. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J. Neurochem. 83, 1509–1524 (2002).
Jin, K. et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl Acad. Sci. USA 101, 343–347 (2004).
Mattson, M. P. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 3, 65–94 (2003).
Ben-Ari, Y. & Cossart, R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 23, 580–587 (2000).
Perl, T. M. et al. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 323, 1631–1633 (1990).
Kisby, G. E., Ellison, M. & Spencer, P. S. Content of the neurotoxins cycasin (methylazoxymethanol β-D-glucoside) and BMAA (β-N-methylamino-L-alanine) in cycad flour prepared by Guam Chamorros. Neurology 42, 1336–1340 (1992).
Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983). First direct evidence that an environmental neurotoxin can cause parkinsonism.
Panov, A. et al. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 280, 42026–42035 (2005).
Ludolph, A. C., He, F., Spencer, P. S., Hammerstad, J. & Sabri, M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 18, 492–498 (1991).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 4, 517–529 (2003).
Toescu, E. C., Verkhratsky, A. & Landfield, P. W. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 27, 614–620 (2004).
Guo, Q. et al. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nature Med. 5, 101–106 (1999).
Schneider, I. et al. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J. Biol. Chem. 276, 11539–11544 (2001).
Martinez, J., Moeller, I., Erdjument-Bromage, H., Tempst, P. & Lauring, B. Parkinson's disease-associated α-synuclein is a calmodulin substrate. J. Biol. Chem. 278, 17379–17387 (2003).
Tang, T. S. et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc. Natl Acad. Sci. USA 102, 2602–2607 (2005).
Smith, R. G. et al. Altered muscle calcium channel binding kinetics in autoimmune motoneuron disease. Muscle Nerve 18, 620–627 (1995).
Von Lewinski, F. & Keller, B. U. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 28, 494–500 (2005).
Mattson, M. P., Rychlik, B., Chu, C. & Christakos, S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron 6, 41–51 (1991).
Magloczky, Z. & Freund, T. F. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience 56, 317–335 (1993).
Guela, C. et al. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J. Comp. Neurol. 455, 249–259 (2003).
Thorns, V., Licastro, F. & Masliah, E. Locally reduced levels of acidic FGF lead to decreased expression of 28-kDa calbindin and contribute to the selective vulnerability of the neurons in the entorhinal cortex in Alzheimer's disease. Neuropathology 21, 203–211 (2001).
Iacopino, A. M. & Christakos, S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc. Natl Acad. Sci. USA 87, 4078–4082 (1990). Evidence that age- and neuron-specific changes in calcium-binding proteins are associated with SNV.
Alexianu, M. E. et al. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann. Neurol. 36, 846–858 (1994).
Ikonomovic, M. D. et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer's disease. Exp. Neurol. 160, 194–204 (1999).
Williams, T. L., Day, N. C., Ince, P. G., Kamboj, R. K. & Shaw, P. J. Calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann. Neurol. 42, 200–207 (1997).
Melov, S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 27, 601–606 (2004).
Petit-Taboue, M. C., Landeau, B., Desson, J. F., Desgranges, B. & Baron, J. C. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage 7, 176–184 (1998).
Masconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 32, 486–510 (2005).
Dagher, A. Functional imaging in Parkinson's disease. Semin. Neurol. 21, 23–32 (2001).
Feigin, A. et al. Metabolic network abnormalities in early Huntington's disease: an [18F]FDG PET study. J. Nucl. Med. 42, 1591–1595 (2001).
Bubber, P., Haroutunian, V., Fisch, G., Blass, J. P. & Gibson, G. E. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann. Neurol. 57, 695–703 (2005).
Smigrodzki, R., Parks, J. & Parker, W. D. High frequency of mitochondrial complex I mutations in Parkinson's disease and aging. Neurobiol. Aging 25, 1273–1281 (2004).
Seong, I. S. et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum. Mol. Genet. 14, 2871–2880 (2005).
Manfredi, G. & Xu, Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion 5, 77–87 (2005).
Brown, M. R., Geddes, J. W. & Sullivan, P. G. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 36, 401–406 (2004).
Murchison, D., Zawieja, D. C. & Griffith, W. H. Reduced mitochondrial buffering of voltage-gated calcium influx in aged rat basal forebrain neurons. Cell Calcium 36, 61–75 (2004).
Kim, G. W. & Chan, P. H. Oxidative stress and neuronal DNA fragmentation mediate age-dependent vulnerability to the mitochondrial toxin, 3-nitropropionic acid, in the mouse striatum. Neurobiol. Dis. 8, 114–126 (2001). Evidence that neuronal vulnerability to mitochondrial toxins increases with advancing age.
Patel, J. R. & Brewer, G. J. Age-related changes in neuronal glucose uptake in response to glutamate and β-amyloid. J. Neurosci. Res. 72, 527–536 (2003).
Klivenyi, P. et al. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J. Neurosci. 20, 1–7 (2000).
Keller, J. N. et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 18, 687–697 (1998).
Bruce-Keller, A. J., Umberger, G., McFall, R. & Mattson, M. P. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 45, 8–15 (1999).
Duan, W. & Mattson, M. P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J. Neurosci. Res. 57, 195–206 (1999).
Niwa, K., Kazama, K., Younkin, S. G., Carlson, G. A. & Iadecola, C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol. Dis. 9, 61–68 (2002).
Lustbader, J. W. et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science 304, 448–452 (2004).
Keller, J. N. et al. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J. Neurosci. 18, 4439–4450 (1998).
Guo, Q., Christakos, S., Robinson, N. & Mattson, M. P. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc. Natl Acad. Sci. USA 95, 3227–3232 (1998).
Panov, A. V. et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nature Neurosci. 5, 731–736 (2002).
Trushina, E. et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol. Cell Biol. 24, 8195–8209 (2004).
Klivenyi, P. et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Med. 5, 347–350 (1999).
Tarnopolsky, M. A. & Beal, M. F. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann. Neurol. 49, 561–574 (2001). Reviews evidence that a cellular energy deficit has a key role in the degeneration of motor neurons in HD and ALS.
Szweda, P. A., Camouse, M., Lundberg, K. C., Oberley, T. D. & Szweda, L. I. Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Ageing Res. Rev. 2, 383–405 (2003).
Gray, D. A. & Woulfe, J. Lipofuscin and aging: a matter of toxic waste. Sci. Aging Knowledge Environ. RE1 2 Feb 2005.
Nixon, R. A. The calpains in aging and aging-related diseases. Ageing Res. Rev. 2, 407–418 (2003).
Bi, X., Yong, A. P., Zhou, J., Gall, C. M. & Lynch, G. Regionally selective changes in brain lysosomes occur in the transition from young adulthood to middle age in rats. Neuroscience 97, 395–404 (2000).
Keller, J. N., Gee, J. & Ding, Q. The proteasome in brain aging. Ageing Res. Rev. 1, 279–293 (2002).
Keller, J. N., Hanni, K. B. & Markesbery, W. R. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech. Ageing Dev. 113, 61–70 (2000).
Lam, Y. A. et al. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proc. Natl Acad. Sci. USA 97, 9902–9906 (2000).
McNaught, K. S., Olanow, C. W., Halliwell, B., Isacson, O. & Jenner, P. Failure of the ubiquitin-proteasome system in Parkinson's disease. Nature Rev. Neurosci. 2, 589–594 (2001).
Sullivan, P. G. et al. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J. Biol. Chem. 279, 20699–20707 (2004).
Bednarski, E., Ribak, C. E. & Lynch, G. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J. Neurosci. 17, 4006–4021 (1997).
Nixon, R. A. et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 64, 113–122 (2005).
Bergamini, E., Cavallini, G., Donati, A. & Gori, Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed. Pharmacother. 57, 203–208 (2003).
Maggio, J. E. et al. Reversible in vitro growth of Alzheimer disease β-amyloid plaques by deposition of labeled amyloid peptide. Proc. Natl Acad. Sci. USA 89, 5462–5466 (1992).
Petkova, A. T. et al. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science 307, 262–265 (2005). Shows that subtle variations in the environment of pathogenic peptides modifies their self-propagating and neurotoxic properties.
Tanzi, R. E., Moir, R. D. & Wagner, S. L. Clearance of Alzheimer's Aβ peptide: the many roads to perdition. Neuron 43, 605–608 (2004).
Goedert, M. Tau gene mutations and their effects. Mov. Disord. 12, S45–S52 (2005).
Stoothoff, W. H. & Johnson, G. V. Tau phosphorylation: physiological and pathological consequences. Biochim. Biophys. Acta 1739, 280–297 (2005).
Montine, T. J., Amarnath, V., Martin, M. E., Strittmatter, W. J. & Graham, D. G. E-4-hydroxy-2-nonenal is cytotoxic and cross-links cytoskeletal proteins in P19 neuroglial cultures. Am. J. Pathol. 148, 89–93 (1996).
Moore, D. J., West, A. B., Dawson, V. L. & Dawson, T. M. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 28, 57–87 (2005).
Singleton, A. B. et al. α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003). The first evidence that a modest increase in the production of α-synuclein is sufficient to cause PD.
Yavich, L., Tanila, H., Vepsalainen, S. & Jakala, P. Role of α-synuclein in presynaptic dopamine recruitment. J. Neurosci. 24, 11165–11170 (2004).
Bates, G. Huntingtin aggregation and toxicity in Huntington's disease. Lancet 361, 1642–1644 (2003).
Fox, J. H. et al. Cystamine increases L-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J. Neurochem. 91, 413–422 (2004).
Wyttenbach, A. et al. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 11, 1137–1151 (2002).
Giffard, R. G. et al. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J. Exp. Biol. 207, 3213–3220 (2004).
Cohen, F. E. & Kelly, J. W. Therapeutic approaches to protein-misfolding diseases. Nature 426, 905–909 (2003).
Zhao, L., Longo-Guess, C., Harris, B. S., Lee, J. W. & Ackerman, S. L. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nature Genet. 37, 974–979 (2005).
Rousseau, E. et al. Targeting expression of expanded polyglutamine proteins to the endoplasmic reticulum or mitochondria prevents their aggregation. Proc. Natl Acad. Sci. USA 101, 9648–9653 (2004).
Kieran, D. et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nature Med. 10, 402–405 (2004). Pharmacological induction of protein chaperones results in therapeutic benefit in a mouse model of ALS.
Siegel, G. J. & Chauhan, N. B. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res. Rev. 33, 199–227 (2000).
Dore, S., Kar, S. & Quirion, R. Insulin-like growth factor I protects and rescues hippocampal neurons against β-amyloid- and human amylin-induced toxicity. Proc. Natl Acad. Sci. USA 94, 4772–4777 (1997).
Counts, S. E. & Mufson, E. J. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J. Neuropathol. Exp. Neurol. 64, 263–272 (2005).
Gash, D. M., Zhang, Z. & Gerhardt, G. Neuroprotective and neurorestorative properties of GDNF. Ann. Neurol. 44, S121–S125 (1998).
Ross, C. A. Huntington's disease: new paths to pathogenesis. Cell 118, 4–7 (2004).
Wilczak, N. & de Keyser, J. Insulin-like growth factor system in amyotrophic lateral sclerosis. Endocr. Dev. 9, 160–169 (2005).
Hattiangady, B., Rao, M. S., Shetty, G. A. & Shetty, A. K. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 195, 353–371 (2005).
Gooney, M., Messaudi, E., Maher, F. O., Bramham, C. R. & Lynch, M. A. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiol. Aging 25, 1323–1331 (2004).
Monti, B., Berteotti, C. & Contestabile, A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus 15, 1041–1049 (2005).
Adlard, P. A., Perreau, V. M. & Cotman, C. W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging 26, 511–520 (2005).
Yurek, D. M. & Fletcher-Turner, A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp. Neurol. 161, 392–396 (2000).
Dore, S., Kar, S., Rowe, W. & Quirion, R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience 80, 1033–1040 (1997).
D'Costa, A. P., Xu, X., Ingram, R. L. & Sonntag, W. E. Insulin-like growth factor-1 stimulation of protein synthesis is attenuated in cerebral cortex of aging rats. Neuroscience 65, 805–813 (1995).
Woods, A. G., Guthrie, K. M., Kurlawalla, M. A. & Gall, C. M. Deafferentation-induced increases in hippocampal insulin-like growth factor-1 messenger RNA expression are severely attenuated in middle aged and aged rats. Neuroscience 83, 663–668 (1998). Evidence that the capacity of brain cells to increase neurotrophic factor signalling is impaired during ageing.
Sonntag, W. E., Ramsey, M. & Carter, C. S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 4, 195–212 (2005).
Grondin, R. et al. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 23, 1974–1980 (2003).
Martinez-Serrano, A., Fischer, W. & Bjorklund, A. Reversal of age-dependent cognitive impairments and cholinergic neuron atrophy by NGF-secreting neural progenitors grafted to the basal forebrain. Neuron 15, 473–484 (1995). The authors show that it is possible to recover age-dependent cognitive deficits by transplantation of neurotrophic factor-producing cells into the basal forebrain.
Lopez-Lopez, C., LeRoith, D. & Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl Acad. Sci. USA 101, 9833–9838 (2004).
Murer, M. G., Yan, Q. & Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol. 63, 71–124 (2001).
Scott, S. A. & Crutcher, K. A. Nerve growth factor and Alzheimer's disease. Rev. Neurosci. 5, 179–211 (1994).
Zuccato, C. et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001). The first evidence that mutant huntingtin perturbs transcriptional regulation of neurotrophic factor production.
Duan, W. et al. Paroxetine retards disease onset and progression in Huntingtin mutant mice. Ann. Neurol. 55, 590–594 (2004).
Dehmelt, L. & Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 6, 204 (2005).
Al-Chalabi, A. & Miller, C. C. Neurofilaments and neurological disease. Bioessays 25, 346–355 (2003).
Trojanowski, J. Q. & Lee, V. M. The role of tau in Alzheimer's disease. Med. Clin. North Am. 86, 615–627 (2002).
Norris, E. H. & Giasson, B. I. Role of oxidative damage in protein aggregation associated with Parkinson's disease and related disorders. Antioxid. Redox Signal. 7, 672–684 (2005).
O'Neill, C. et al. Dysfunctional intracellular calcium homoeostasis: a central cause of neurodegeneration in Alzheimer's disease. Biochem. Soc. Symp. 67, 177–194 (2001).
Oddo, S. et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003).
Gauthier, L. R. et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 (2004).
Zhang, B., Tu, P., Abtahian, F. & Trojanowski, J. Q. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J. Cell Biol. 139, 1307–1315 (1997). Reports direct evidence that a mutation that causes ALS impairs axonal transport in motor neurons.
Hutton, M. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Ishihara, T. et al. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 24, 751–762 (1999).
Brandt, R., Hundelt, M. & Shahani, N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim. Biophys. Acta 1739, 331–354 (2005).
McGeer, P. L. & McGeer, E. G. Local neuroinflammation and the progression of Alzheimer's disease. J. Neurovirol. 8, 529–538 (2002).
Dickson, D. W., Lee, S. C., Mattiace, L. A., Yen, S. H. & Brosnan, C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia 7, 75–83 (1993).
Eikelenboom, P., Hack, C. E., Rozemuller, J. M. & Stam, F. C. Complement activation in amyloid plaques in Alzhiemer's dementia. Virchows Arch. 56, 259–262 (1989).
Itagaki, S., McGeer, P. L. & Akiyama, H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer's disease brain tissue. Neurosci. Lett. 91, 259–264 (1988).
Eikelenboom, P. & Stam. F. C. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. (Berl.) 57, 239–242 (1982).
Nath, A. et al. Autoantibodies to amyloid β-peptide (Aβ) are increased in Alzheimer's disease patients and Aβ antibodies can enhance Aβ neurotoxicity: implications for disease pathogenesis and vaccine development. Neuromolecular Med. 3, 29–39 (2003).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999). The first evidence that the immune system can be activated to specifically remove amyloid from the brain, suggesting a potential for immunization therapies in the treatment of AD.
Teismann, P. & Schulz, J. B. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 318, 149–161 (2004).
Sargsyan, S. A., Monk, P. N. & Shaw, P. J. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia 51, 241–253 (2005).
Smith, R. G. et al. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc. Natl Acad. Sci. USA 91, 3393–3397 (1994).
Miller, R. G., Mitchell, J. D., Lyon, M. & Moore, D. H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph. Lateral Scler. Other Motor Neuron Disord. 4, 191–206 (2003).
Reisberg, B. et al. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch. Neurol. 63, 49–54 (2006).
Wang, J. et al. Caloric restriction attenuates β-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 19, 659–661 (2005).
Lazarov, O. et al. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell 120, 701–713 (2005). Reports the first evidence that exercise/cognitive stimulation can enhance clearance of Aβ from the brain.
Lim, G. P. et al. A diet enriched with the ω-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 25, 3032–3040 (2005).
DeKosky, S. T. Statin therapy in the treatment of Alzheimer disease: what is the rationale? Am. J. Med. 118, S48–S53 (2005).
Lipton, S. A. Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders. J. Alzheimers Dis. 6, S61–S74 (2004).
Duan, W. et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 52, 597–606 (2002).
Bae, B. I. et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron 47, 29–41 (2005).
Ellis, A. C. & Rosenfeld, J. The role of creatine in the management of amyotrophic lateral sclerosis and other neurodegenerative disorders. CNS Drugs 18, 967–980 (2004).
Kordower, J. H. et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773 (2000). Reports the efficacy of gene therapy in a non-human primate model of PD.
Dobrowolny, G. et al. Muscle expression of a local IGF-1 isoform protects motor neurons in an ALS mouse model. J. Cell Biol. 168, 193–199 (2005).
Levy, Y. S., Gilgun-Sherki, Y., Melamed, E. & Offen, D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs 19, 97–127 (2005).
Gelinas, D. S., DaSilva, K., Fenili, D., St Georger-Hyslop, P. & McLaurin, J. Immunotherapy for Alzheimer's disease. Proc. Natl Acad. Sci. USA 101, S14657–S14662 (2004).
Harper, S. Q. et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc. Natl Acad. Sci. USA 102, 5820–5825 (2005).
Logroscino, G. et al. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann. Neurol. 39, 89–94 (1996).
Luchsinger, J. A., Tang, M. X., Shea, S. & Mayeux, R. Caloric intake and the risk of Alzheimer disease. Arch. Neurol. 59, 1258–1263 (2002).
Luchsinger, J. A. & Mayeux, R. Dietary factors and Alzheimer's disease. Lancet Neurol. 3, 579–587 (2004).
Mattson, M. P. & Shea, T. B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 26, 137–146 (2003).
Carro, E., Trejo, J. L., Busiguina, S. & Torres-Aleman, J. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 21, 5678–5684 (2001).
Laurin, D., Verrault, R., Lindsay, J., MacPherson, K. & Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 58, 498–504 (2001).
Mattson, M. P. & Wan, R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 16, 129–137 (2005).
Jeffery, B., Barlow, T., Moizer, K., Paul, S. & Boyle, C. Amnestic shellfish poison. Food Chem. Toxicol. 42, 545–557 (2004).
Lee, J., Bruce-Keller, A. J., Kruman, Y., Chan, S. L. & Mattson, M. P. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J. Neurosci. Res. 57, 48–61 (1999).
Di Monte, D. A. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2, 531–538 (2003).
Lee, J. M., Zipfel, G. J. & Choi, D. W. The changing landscape of ischaemic brain injury mechanisms. Nature 399 (Suppl. 6738), A7–A14 (1999).
Youdim, K. A. & Joseph, J. A. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic. Biol. Med. 30, 583–594 (2001).
Di Matteo, V. & Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Curr. Drug Targets CNS Neurol. Disord. 2, 95–107 (2003).
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Selective neuronal vulnerability
-
(SNV). The susceptibility of specific populations of neurons that is limited to a region or regions of the nervous system.
- Proteasome
-
A protein complex responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin.
- Lipid peroxidation
-
An autocatalytic process in which free radicals attack double bonds in membrane lipids, resulting in structural damage to membranes and the liberation of toxic aldehydes such as 4-hydroxynonenal.
- Autophagy
-
A process in which damaged organelles are degraded within membrane-bound organelles.
- Dietary restriction
-
A decrease in the amount of food consumed over time (caloric restriction) and/or the frequency of meals (intermittent fasting).
- Reactive oxygen species
-
(ROS). Highly reactive oxygen-based molecules with an unpaired electron in their outer orbital that are capable of damaging proteins, lipids and nucleic acids. Examples include hydrogen peroxide and hydroxyl radicals.
- Hormesis
-
A process in which exposure of a cell or organism to a sublethal level of stress increases the resistance of that cell or organism to a subsequent higher and otherwise lethal level of the same or different stress.
- Sirtuins
-
A family of histone deacetylases that have important roles in cellular stress responses and energy metabolism.
- Mitochondrial uncoupling proteins
-
A family of proteins that reside in the mitochondrial inner membrane and promote a proton leak across the membrane, thereby decreasing oxidative phosphorylation and reactive oxygen species production.
- Trans-entorhinal region
-
An area of the brain — located between association cortices and the hippocampus — that is important in the integration of information and learning and memory processes.
- Caspases
-
A family of cysteine proteases that cleave proteins at specific aspartate residues and have a key role in inflammation and mammalian apoptosis.
- BCL2
-
A protein that promotes the survival of neurons by stabilizing mitochondrial membranes and decreasing oxidative stress.
- Calpains
-
Cysteine proteases activated by calcium that cleave various substrates, including cytoskeletal proteins.
- Permeability transition pores
-
Pores in the mitochondrial membranes that are formed by proteins in response to signals that trigger apoptosis.
- Ceramides
-
Membrane lipids that are incorporated into sphingomyelin and are released in response to the activation of sphingomyelinases.
- Ion-motive ATPases
-
Energy-dependent ion pumps in membranes that are essential for the restoration and maintenance of the Na+ and Ca2+ gradients.
- Afterhyperpolarization
-
The membrane hyperpolarization that follows the occurrence of an action potential.
- Glutathione peroxidase
-
An antioxidant enzyme that converts hydrogen peroxide to water.
- Mitochondrial manganese superoxide dismutase
-
An antioxidant enzyme located in mitochondria that converts superoxide anion radicals to hydrogen peroxide.
- Lysosome
-
A membrane-bound organelle with a low pH that contains high concentrations of enzymes that degrade proteins.
- Frontotemporal dementia
-
A neurodegenerative disorder resulting from the degeneration of neurons in the frontal lobe.
- Repeat isoform
-
An isoform of the microtubule-associated protein tau that contains either three or four microtubule-binding domains.
- Cytokines
-
A large class of intercellular signalling proteins that are important in neural–immune system interactions and inflammatory processes.
- Complement factors
-
Proteins that function in innate immunity, often forming pores in membranes, which results in cell death.
- Leukocyte adhesion molecules
-
Proteins located on the surface of vascular endothelial cells that bind to leukocytes, thereby facilitating the passage of the leukocytes across the blood–brain barrier.
Rights and permissions
About this article
Cite this article
Mattson, M., Magnus, T. Ageing and neuronal vulnerability. Nat Rev Neurosci 7, 278–294 (2006). https://doi.org/10.1038/nrn1886
Issue Date:
DOI: https://doi.org/10.1038/nrn1886
This article is cited by
-
Human lifespan and sex-specific patterns of resilience to disease: a retrospective population-wide cohort study
BMC Medicine (2024)
-
Mucosal TLR5 activation controls healthspan and longevity
Nature Communications (2024)
-
Presenilins regulate synaptic plasticity in the perforant pathways of the hippocampus
Molecular Brain (2023)
-
JAK-STAT signaling in inflammation and stress-related diseases: implications for therapeutic interventions
Molecular Biomedicine (2023)
-
REM sleep is associated with the volume of the cholinergic basal forebrain in aMCI individuals
Alzheimer's Research & Therapy (2023)